建立以紅外線活化的激酶-上轉換粒子平台以控制細胞內信號傳遞
Construction of a Near-Infrared-Activatable Enzyme Platform to Remotely Trigger Intracellular Signal Transduction Using an Upconversion NanoparticleACS NANO 2015, 9, 7041
Hua-De Gao, Pounraj Thanasekaran, Chao-Wei Chiang, Jia-Lin Hong, Yen-Chun Liu, Yu-Hsu ChangHsien-Ming Lee
利用光來活化生物分子可以達到遙控的目的並且有極高的時間及空間上的控制性, 是化學生物學家重要的實驗工具。但是傳統的方法需要以紫外線光照,因此在進行細胞實驗時,無法屏除來自紫外線對生物的傷害及干擾。此研究利用鑭系元素的上轉換粒子與負責磷酸化的激酶交聯之後,成功的達成用紅外線來控制光化學生物學(信號傳遞分子磷酸化)反應,並進一步控制生物上現象,如細胞行為。此一平台也提供了另一波長來控制光化學反應,使得在活體內可進行兩種光化學反應,這些都是以前傳統紫外光光解化學所做不到的。
Photoactivatable (caged) bio-effectors provide a way to re-motely trigger or disable biochemical pathways in living organisms at a desired time and location with a pulse of light (uncaging), but the phototoxicity of ultraviolet (UV) often limits its application. In this study, we have demonstrated the near-infrared (NIR) photoactivatable enzyme platform using protein kinase A (PKA), an important enzyme in cell biology. We successfully photoactivated PKA using NIR to phosphorylate its substrate, and this induced a downstream cellular response in living cells with high spatiotemporal resolution. In addition, this system allows NIR to selectively activate the caged enzyme immobilized on the nanoparticle surface without activating other caged proteins in the cytosol. This NIR-responsive enzyme-nanoparticle system provides an innovative approach to remote-control proteins and enzymes, which can be used by researchers who need to avoid direct UV irradiation or use UV as a secondary channel to turn on a bio-effector.
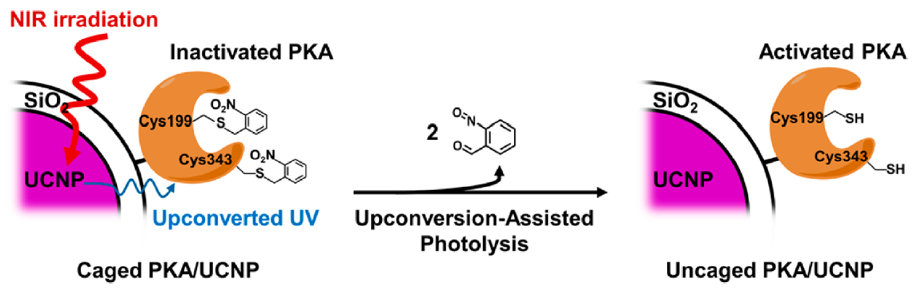
Figure 1. Crosslinked caged kinase / nanoparticle, upon the NIR irradiation, can upconvert the NIR to UV, and activate the caged kinase on its surface to restore its kinase activity.
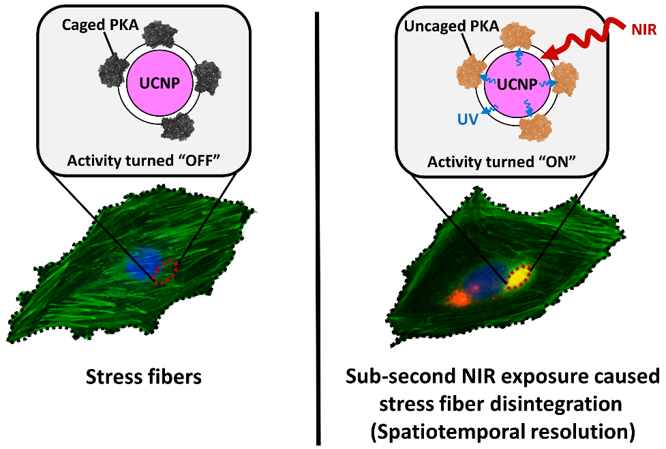
Figure 2. The spatial resolution of this technique can reach to mm level (yellow region shows stress fiber unbundled by NIR light.
