可增進燃料電池效能的三銅金屬簇功能化碳電極
Tricopper Cluster Complex Functionalized Carbon Electrode: A Strategy to Overcome the Overpotential and Production of Hydrogen Peroxide in the Oxygen Reduction ReactionAngew. Chem., Int. Ed. 2018, 57, 3612-3616. Natarajan Thiyagarajan, Damodar Janmanchi, Yi-Fang Tsai, Wondemagegn Hailemichael Wanna, Ravirala Ramu, Sunney I. Chan*, Jyh-Myng Zen*, and Steve S.-F. Yu*
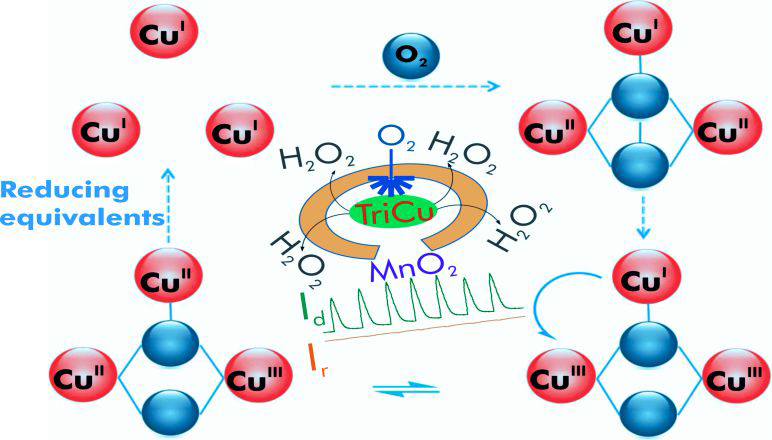
氧氣還原反應是最重要的基本化學反應之一,在生物系統中,以四個電子,將氧分子還原為水,是細胞中進行有氧呼吸的重要關鍵。在技術上,這個反應也是氫/氧燃料電池中,兩個最重要的路徑之一,且其陰極的反應路徑,亦會影響燃料電池的效能。一般而言,鉑貴重金屬,具備最低的過電位,常被應用來做為電極。然而,鉑電極受限於緩慢的動力學模式與高單價,大尺度地使用鉑金屬既不實際亦不可行。基於此項關鍵研究的挑戰,研發新的材料來製備電極進行氧氣還原反應,為重要的研發課題。在此,我們報導一個新穎的三銅金屬簇,經高度分散於經電化學還原之碳黑基材後,塗佈於網印碳電極的表面上,以應用於研究氧氣還原的過程;在pH 13與7,其氧氣還原電位經測量分別為 ~0.92 與 ~0.77伏,除了最低電位的鉑與其他貴金屬外,與目前任何相關之含銅錯合物的最佳數值相當。由動力學的研究顯示,此三銅金屬簇碳電極可有效率將氧分子透過四個電子的還原,轉換成水,僅有極少量的過氧化氫生成。我們也利用雙銅單銀的三核過渡金屬簇與僅具三銅的催化劑,進一步比較氧氣活化的效能,擁有三個還原當量的催化劑,可有效率地降低其經四個電子還原所可能面臨的過電位,並抑低過氧化氫的產生。因此,快速轉移三個電子至氧分子,在氧氣還原反應中,可有效地進行氧-氧鍵的斷鍵。
The oxygen reduction reaction (ORR) is one of the fundamental reactions in chemistry. In biology, the 4-e− reduction of dioxygen (O2) to water (H2O) is key to cellular respiration of aerobic organisms and in biological oxidations. In enzymes mediating this process, the reaction is facile and highly efficient. Technologically, this reaction is also one of the two most important processes in the H2/O2 fuel cell. Here, the cathodic process affects the efficiency of the fuel cell. Platinum is typically employed as the electrode as the over-potential is the lowest with this noble metal. However, the Pt electrode exhibits sluggish ORR kinetics and the cost, large-scale usage of Pt is neither practical nor viable. For this reason, there is considerable impetus in developing novel materials to fabricate electrodes for the ORR. In this work, we have reported on a study of the ORR on a screen printed carbon electrode (SPCE) surface mediated by the tricopper cluster complex Cu3(7-N-Etppz(CH2OH)) dispersed on electrochemically reduced carbon black, where 7-N-Etppz(CH2OH) is the ligand 3,3'-(6-(hydroxymethyl)-1,4-diazepane-1,4-diyl)bis(1-(4-ethylpiperazin-1-yl) propan-2-ol). Onset oxygen reduction potentials of ~0.92 V and ~0.77 V are observed at pH 13 and 7 vs. the reversible hydrogen electrode (RHE). Based on half wave potentials (E1/2), the corresponding over-potentials are ~0.42 V and ~0.68 V, respectively, which are among the lowest except for Pt and other noble metals. Kinetic studies indicate that the trinuclear copper catalyst can accomplish the 4-e- reduction of O2 efficiently and the ORR is accompanied by the production of only small amounts of H2O2. The involvement of the copper triad in the O2 activation process is verified by comparing the performances between electrodes modified by a Cu3 and Cu2Ag catalyst, underscoring the significance of having three reducing equivalents readily available for ORR to alleviate the over-potential as well as production of H2O2. Thus, the rapid delivery of three electrons to the O2 molecule is essential for the efficient cleavage of the O–O bond during ORR.