同碳雙碳烯 : 與小分子反應時出乎意料的π-電子接受能力
Carbodicarbenes: Unexpected π‑Accepting Ability during Reactivity with Small MoleculesJ. Am. Chem. Soc. 2017, 139, 12830−12836. Wen-Ching Chen,* Wei-Chih Shih, Titel Jurca, Lili Zhao, Diego M. Andrada, Chun-Jung Peng, Chun-Chi Chang, Shu-kai Liu, Yi-Ping Wang, Yuh-Sheng Wen, Glenn P. A. Yap, Chao-Ping Hsu, Gernot Frenking,* and Tiow-Gan Ong*
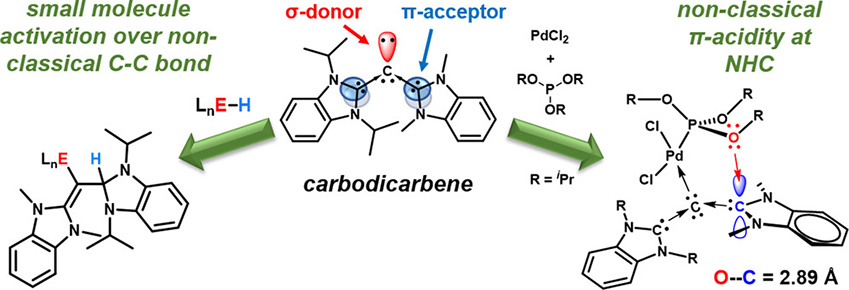
同碳雙碳烯是碳化物配位基家族中較少被探索的成員,但是近年來的研究發現其具有特殊的電子特徵及伴隨的反應性。利用其與硼烷及矽烷等小分子進行反應,可以得到1,2-加成的產物,結合其鈀金屬錯合物之X-ray晶體結構研究,可以推測其中心碳原子配位於小分子或金屬化合物時,旁邊的苯並咪唑NCN的碳原子會形成π-電子接受中心。這種具備未預料之π-酸性的同碳雙碳烯,其與小分子之間的特有反應性,與FLP (frustrated Lewis pair) 的性質極為相近。
An investigation of carbodicarbenes, the less explored member of the carbenic complex/ligand family has yielded unexpected electronic features and concomitant reactivity. Observed 1,2-addition of E−H bonds (E = B, C, Si) across the carbone central carbon and that of the flanking N-heterocyclic carbene (NHC) fragment, combined with single-crystal X-ray studies of a model Pd complex strongly suggests a significant level of π-accepting ability at the central carbon of the NHC moiety. This feature is atypical of classic NHCs, which are strong σ-donors, with only nominal π-accepting ability. The unanticipated π-acidity is critical for engendering carbodicarbenes with reactivity more commonly observed with frustrated Lewis pairs (FLPs) rather than the more closely related NHCs and cyclic (alkyl)(amino)carbenes (CAACs).