Institute of Chemistry, Academia Sinica – People

Charge-induced intramolecular charge transfer and its applications.
Molecular design of organic semiconductors.
Supramolecular chemistry and self-assembly.
Molecular structures and reaction mechanisms.
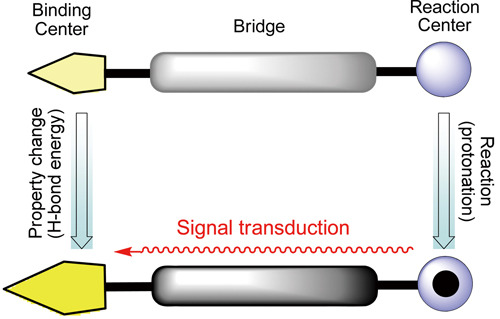
Remote control of hydrogen bonds can be achieved with the use of pi-conjugated molecules bearing a binding center, a bridge, and a reaction center. When reactions take place at the reaction center, the binding ability of the binding center varies accordingly.
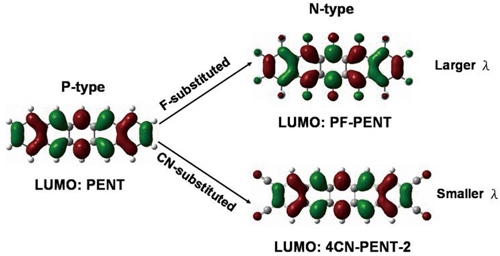
N-type organic semiconductors can be obtained by substituting electron withdrawing groups to p-type frameworks. F-substituted system has larger internal reorganization energy due to the additional contribution of C-F bond. However, CN-substituted system has smaller reorganization energy by extending electron delocalization in a nonbonding fashion.
- Chen, W.-C.; Chao, I. Charge Transport Properties of Open-Shell Graphene Fragments: A Computational Study of the Phenalenyl Tilings. Phys. Chem. Chem. Phys 2021, 23, 3256.
- Chen, Z.-J.; Lu, H.-F.; Chiu, C.-W.; Hou, F.-M.; Matsunaga, Y.; Chao, I.; Yang, J.-S. A Molecular Rotor That Probes the Helical Inversion of Stiff-Stilbene. Org. Lett. 2020, 22, 9158.
- Chen, W.-C.; Chao, I. Molecular Orbital-Based Design of π-Conjugated Organic Materials with Small Internal Reorganization Energy: Generation of Nonbonding Character in Frontier Orbitals. J. Phys. Chem. C 2014, 118, 20176.
- Peng, W.-T.; Chang, Y.-C.; Chao, I. A Design Strategy for Motion Control Systems with Identical Binding Sites. ChemPhysChem 2013, 14, 500.
- Li, W.-S.; Wang, S.-C.; Hwang, T.-S.; Chao, I. Substituent Effect on the Structural Behavior of Modified Cyclodextrin: A Molecular Dynamics Study on Methylated β-CDs. J. Phys. Chem. B 2012, 116, 3477.
- Kao, C.-Y.; Hsu, Y.-T.; Lu, H.-F.; Chao, I.; Huang, S.-L.; Lin, Y.-C.; Sun, W.-T.; Yang, J.-S. Toward a Four-Toothed Molecular Bevel Gear with C2-Symmetrial Rotors. J. Org. Chem. 2011, 76, 5782.
- Chang, Y.-C.; Kuo, M.-Y.; Chen, C.-P.; Lu, H.-F.; Chao, I. On the Air-Stability of n- Channel Organic Field-Effect Transistors: A Theoretical Study of Adiabatic Electron Affinities of Organic Semiconductors. J. Phys. Chem. C 2010, 114, 11595.
- Chang, Y.-C.; Chao, I. An Important Key to Design Molecules with Small Internal Reorganization Energy: Strong Nonbonding Character in Frontier Orbitals. J. Phys. Chem. Lett. 2010, 1, 116.
- Chang, Y.-C.; Chen, Y.-D.; Chen, C.-H.; Wen, Y.-S.; Lin, J. T.; Chen, H.-Y.; Kuo, M.- Y.; Chao, I. Crystal Engineering for π−π Stacking via Interaction between Electron- Rich and Electron-Deficient Heteroaromatics. J. Org. Chem. 2008, 73, 4608.
- Kuo, M.-Y.; Chen, H.-Y.; Chao, I. Cyanation: Providing Three-in-One Advantage for the Design of n-Type Organic Field-Effect Transistors? Chem. Eur. J. 2007, 13, 4750.
- Chen, H.-Y.; Chao, I. Toward Rational Design of Functionalized Pentacenes: Reduction of the Impact of Functionalization on Reorganization Energy. ChemPhysChem 2006, 7, 2003.
- Lo, S.-J.; Li, W.-S.; Chen, Y.-H.; Chao, I. Theoretical Study of Remote Control of Hydrogen Bond Strengths in Donor-Bridge-Acceptor Systems: Principles for Designing Effective Bridges with Substituent Tuning. Chem. Eur. J. 2005, 11, 6533.
- Chen, H.-Y.; Chao, I. Effect of Perfluorination on the Charge-Transport Properties of Organic Semiconductors: Density Functional Theory Study of Perfluorinated Pentacene and Sexithiophene. Chem. Phys. Lett. 2005, 401, 539.
- Hwang, T.-S.; Juan, N.; Chen, H.-Y.; Chen, C.-C.; Lo, S.-J.; Chao, I. Control of Hydrogen-Bond Strengths via Push-Pull Effects Triggered by a Remote Reaction Center: A Theoretical Study. Chem. Eur. J. 2004, 10, 1616.
- Chao, I.; Hwang, T.-S. Remote Communication between Charge Centers and Hydrogen-Bonding Sites: Possibility for a Signal Transducer. Angew. Chem. Int. Ed. 2001, 40, 2703.
- Li, T.-W.; Chao, I.; Tao, Y.-T. The Relationship between Packing Structures and Head Groups of Self-Assembled Monolayers on Au(111): Bridging Experimental Observations through Computer Simulations. J. Phys. Chem. B 1998, 102, 2935.
- Chao, I.; Lu, H.-F.; Chou, T.-S. A Theoretical Study of the Exceptional Thermal Reactivity of 2-Phenyloxazolo-3-sulfolene: Roles Played by Aromaticity and Strain. J. Org. Chem. 1997, 62, 7882.
- Chao, I.; Chen, J.-C. Resolving the Puzzling Eclipsed Conformation of the Methyl Group in a Tricyclic Orthoamide Trihydrate. Angew. Chem. Int. Ed. Engl. 1996, 35, 195.
科普文章:
Update: 2023-11-08
- Chen, W.-C.;* Chao, I.* Charge Transport Properties of Open-Shell Graphene Fragments: A Computational Study of the Phenalenyl Tilings. Physical Chemistry Chemical Physics 2021, 23, 3256-3266.
- Ferrins, L.; Chao, I.; and Cesa, M Stakeholders’ Thoughts on the Future of IUPAC. Chemistry International 2021, 43, 36-39.
- Chen Zi-Jian, Lu Hsiu-Feng, Chiu Chun-Wei, Hou Fen-Miao, Matsunaga Yuki, Chao Ito*, Yang Jye-Shane* A Molecular Rotor That Probes the Helical Inversion of Stiff-Stilbene. Organic Letters 2020, 22(23) 9158-9162.
- Ting-Yu Li, Yi-Chun Lin, Yu-Huei Song, Hsiu-Feng Lu, Ito Chao, Chih-Hsiu Lin* Synthesis and Physical Study of Perylene and Anthracene Polynitrile as Electron Acceptors. ORGANIC LETTERS 2019-07, 21(14), 5397-5401.
- Jhih-Liang Huang, BillaBhargava Rao, Manyam Praveen Kumar, Hsiu-Feng Lu, Ito Chao, Chih-Hsiu Lin* Dicyclopenta[ghi,pqr]perylene as a Structural Motif for Bowl-Shaped Hydrocarbons: Synthetic and Conformational Studies. ORGANIC LETTERS 2019-04, 21(8), 2504-2508.
- Tian-You Cheng, Jiun-Haw Lee, Chia-Hsun Chen, Po-Hsun Chen, Po-Sheng Wang, Chuan-En Lin, Bo-Yen Lin, Yi-Hsin Lan, Yu-Hsuan Hsieh, Jau-Jiun Huang, Hsiu-Feng Lu, Ito Chao, Man-kit Leung,* Tien-Lung Chiu,* Chi-Feng Lin* Carrier Transport and Recombination Mechanism in Blue Phosphorescent Organic Light-Emitting Diode with Hosts Consisting of Cabazole- and Triazole-Moiety. SCIENTIFIC REPORTS 2019-03, 9, 3654-3665.
- Hsiu-Feng Lu, Hui-Fen Chen, Chai-Lin Kao, Ito Chao, Hsing-Yin Chen* A Computational Study of the Fenton Reaction in Different pH Ranges. PHYSICAL CHEMISTRY CHEMICAL PHYSICS 2018-08, 20, 22890-22901.
- Huang Ding-Chi, Kuo Chi-Hsien, Ho Man-Tzu, Lin Bo-Chao, Peng Wei-Tao, Chao Ito, Hsu Chao-Ping, Tao Yu-Tai Contorted tetrabenzoacenes of varied conjugation: charge transport study with single-crystal field-effect transistors. Journal of Materials Chemistry C 2017, 5(31), 7935-7943.
- Tseng Ting, Lu Hsiu-Feng, Kao Chen-Yi, Chiu Chun-Wei, Chao Ito, Prabhakar Chetti, Yang Jye-Shane Redox-Gated Tristable Molecular Brakes of Geared Rotation. The Journal of Organic Chemistry 2017, 82(10), 5354-5366.
- Cheng-Hua Lee, Hung-Yu Huang, Jey-Jau Lee, Chia-Yuan Huang, Ya-Chuan Kao, Gene-Hsiang Lee, Shie-Ming Peng, Jyh-Chiang Jiang, Ito Chao,* Kuang-Lieh Lu* Amide-CO2 Interaction Induced Gate-Opening Behavior for CO2 Adsorption in 2-Fold Interpenetrating Framework. ChemistrySelect 2016-07, 1, 2923 – 2929.
- Rofeamor P. Oben, Mei-Chun Tseng, Indah Primadona, Jun Hsiao, I-Che Li, Rey Y. Capangpangan, Hsiu-Fong Lu, Wan-Sheung Li, Ito Chao, Chun-Cheng Lin, Yu-Ju Chen* Chemical Science UV-activated multilayer nanomatrix provides one-step tunable carbohydrate structural characterization in MALDI-MS. Chemical Science 2015, 6, 4790-4800.
- Chen-Yi Kao, Hsiu-Feng Lu, Ito Chao*, Jye-Shane Yang* A Rotary Molecuar Motor Gated by Electrical Energy. ORGANIC LETTERS 2014-11, 16, 6100-6103.
- Wei-Chih Chen, Ito Chao* Molecular Orbital-Based Design of pi-Conjugated Organic Materials with Small Internal Reorganization Energy: Generation of Nonbonding Character in Frontier Orbitals. Journal of Physical Chemistry C 2014-08, 118, 20176-20183.
- Ming-Chou Chen*, Sureshraju Vegiraju, Chi-Ming Huang, Peng-Yi Huang, Kumaresan Prabakaran, Shueh Lin Yau, Wei-Chih Chen, Wei-Tao Peng, Ito Chao*, Choongik Kim* and Yu-Tai Tao* Asymmetric Fused Thiophenes for Field-Effect Transistors: Crystal Structure-Film Microstructure-Transistor- Performance Correlations. JOURNAL OF MATERIALS CHEMISTRY C 2014-06, 2, 8892-8902.
- Chi-Hsien Kuo, Ding-Chi Huang, Wei-Tao Peng, Kenta Goto, Ito Chao*, Yu-Tai Tao* Substituent Effect on the Crystal Packing and Electronic Coupling of Tetrabenzocoronenes: A Structure-Property Correlation. JOURNAL OF MATERIALS CHEMISTRY C 2014-05, 2, 3928-3935.
- Cheng-Hua Lee, Hung-Yu Huang, Yen-Hsiang Liu, Tzuoo-Tsair Luo, Gene-Hsiang Lee, Shie-Ming Peng, Jyh-Chiang Jiang, Ito Chao,* Kuang-Lieh Lu* Cooperative Effect of Unsheltered Amide Groups on CO2 Adsorption Inside Open-Ended Channels of a Zinc(II)-Organic Framework. INORGANIC CHEMISTRY 2013-03, 52, 3962–3968.
- Wei-Tao Peng, Yu-Chang Chang, Ito Chao* A Design Strategy for Motion Control Systems with Identical Binding Sites. CHEMPHYSCHEM 2013-02, 14, 500-504.
- Someshwar Pola, Chi-Hsien Kuo, Wei-Tao Peng, Md. Minarul Islam, Ito Chao,* Yu-Tai Tao* Contorted Tetrabenzocoronene Derivatives for Single Crystal Field Effect Transistors: Correlation between Packing and Mobility. CHEMISTRY OF MATERIALS 2012-07, 24, 2566-2571.
- Kuo Yuan Chiu, Yi-Jung Tu, Chia-Jung Lee, Te-Fang Yang*, Long-Li Lai, Ito Chao*, Yuhlong Oliver Su* Unusual Spectral and Electrochemical Properties of Azobenzene-Substituted Porphyrins. ELECTROCHIMICA ACTA 2012-02, 62, 51-62.
- Wan-Sheung Li, San-Chi Wang, Tsong-Song Hwang, Ito Chao* Substituent Effect on the Structural Behavior of Modified Cyclodextrin: A Molecular Dynamics Study on Methylated beta-CDs. JOURNAL OF PHYSICAL CHEMISTRY B 2012-02, 116, 3477-3489.
- Yi-Jung Tu, Hsu Chun Cheng, Ito Chao*, Cheng-Ru Cho, Ru-Jen Cheng*, Yuhlong Oliver Su* Intriguing Electrochemical Behavior of Free Base Porphyrins: Effect of Porphyrin-meso-Phenyl Interation Controlled by Position of Substituents on meso-Phenyls. JOURNAL OF PHYSICAL CHEMISTRY A 2012-02, 116, 1632-1637.
- Hau-Nan Lee, Ito Chao, Tzu-Min Su* Asymmetry in the Internal Energies of the Optical Rotamers of 1-Bromo-2-Chloroethane in Oriented-Molecule/Surface Scattering: A Classical Molecular Dynamics Study. CHEMICAL PHYSICS LETTERS 2011-12, 517(4-6), 132-138.
- Gao-Fong Chang, Chun-Hung Wang, Hung-Chieh Lu, Lou-Sing Kan, Ito Chao, Wei Hao Chen, Anil Kumar, Liyang Lo, Mira Anne C. dela Rosa, Chen-Hsiung Hung* Factors That Regulate the Conformation of m-Benziporphodimethene Complexes: Agostic Metal–Arene Interaction, Hydrogen Bonding, and 2 π Coordination. CHEMISTRY-A EUROPEAN JOURNAL 2011-09, 17(40), 11332-11343.
- Chen-Yi Kao, Ya-Ting Hsu, Hsiu-Feng Lu, Ito Chao*, Shou-Ling Huang, Ying-Chih Lin, Wei-Ting Sun, Jye-Shane Yang* Toward a Four-Toothed Molecular Bevel Gear with C2-Symmetrical Rotors. JOURNAL OF ORGANIC CHEMISTRY 2011-05, 76(14), 5782-5792.
- Md. Minarul Islam, Famil Valiyev, Hsiu-Feng Lu, Ming-Yu Kuo, Ito Chao*, Yu-Tai Tao* High Performance Single-Crystal Field-Effect Transistors Based on Twisted Polyaromatic Semiconductor Pyreno[4,5-a]coronene. CHEMICAL COMMUNICATIONS 2011-02, 47, 2008-2010.
- Ying-Chen Chen, Wei-Ting Sun, Hsiu-Feng Lu, Ito Chao*, Guan-Jhih Huang, Ying-Chih Lin, Shou-Ling Huang, Hsin-Hau Huang,Yan-Duo Lin, Jye-Shane Yang* A Pentiptycene-Derived Molecular Brake: Photochemical E->Z and Electrochemical Z->E Switching of an Enone Module. CHEMISTRY-A EUROPEAN JOURNAL 2011-01, 17, 1193-1200.
- Wei-Ting Sun, Yau-Ting Huang, Guan-Jhih Huang, Hsiu-Feng Lu, Ito Chao*, Shou-Ling Huang, Shing-Jong Huang, Ying-Chih Lin, Jinn-Hsuan Ho, Jye-Shane Yang* Pentiptycene-Derived Light-Driven Molecular Brakes: Substituent Effects of the Brake Component. CHEMISTRY-A EUROPEAN JOURNAL 2010-10, 16, 11594-11604.
- Yu-Chang Chang, Ming-Yu Kuo, Chih-Ping Chen, Hsiu-Feng Lu, Ito Chao* On the Air Stability of n-Channel Organic Field-Effect Transistors: A Theoretical Study of Adiabatic Electron Affinities of Organic Semiconductors. Journal of Physical Chemistry C 2010-06, 114(26), 11595-11601.
- Yu-Chang Chang, Ito Chao* An Important Key to Design Molecules with Small Internal Reorganization Energy: Strong Nonbonding Character in Frontier Orbitals. J. Phys. Chem. Lett. 2010-01, 1(1), 116-121.
- Ling-Yung Wang, I-Hung Chiang, Po-Jen Yang, Wan-Sheung Li, I-To Chao, Hong-Cheu Lin* "Configuration Effects of H-Bonded Sites and Rigid Core Lengths on H-Bonded Banana-Shaped Liquid Crystalline Supramolecules Consisting of Symmetric Trimers and Asymmetric Hetero-Dimers. J. Phys. Chem. B 2009-11, 113(44), 14648-14660.
- Shou-Zheng Weng, Paritosh Shukla, Ming-Yu Kuo, Yu-Chang Chang, Hwo-Shuenn Sheu, Ito Chao*, Yu-Tai Tao* Diazapentacene Derivatives as Thin-Film Transistor Materials: Morphology Control in Realizing High-Field-Effect Mobility. ACS Applied Materials & Interfaces 2009-09, 1(9), 2071-2079.
- Yi-Ping Huang, Chung-Chih Tsai, Wei-Chih Shih, Yu-Chang Chang, Shen-Ta Lin, Glenn P. A. Yap, Ito Chao*, Tiow-Gan Ong* Kinetic and Thermodynamic Study of Syn-Anti Isomeriztaion of Nickel Complexes Bearing Amino-Linked N-Heterocyclic Carbene Ligands: The Effect of the Pendant Arm of the NHC. Organometallics 2009-08, 28(15), 4316-4323.
- Chun-Ju Chuang, Wan-Sheung Li, Chien-Chen Lai, Yi-Hung Liu, Shie-Ming Peng, Ito Chao*, Sheng-Hsien Chiu* A Molecular Cage-Based [2]Rotaxane That Behaves as a Molecular Muscle. ORGANIC LETTERS 2009-01, 11(2), 385-388.
- J.-S. Yang*, Y.-T. Huang, J.-H. Ho, W.-T. Sun, H.-H. Huang, Y.-C. Lin*, S.-J. Huang, S.-L. Huang, H.-F. Lu, I. Chao* A Pentiptycene-Derived Light-Driven Molecular Brake. ORGANIC LETTERS 2008-05, 10, 2279-2282.
- Y.-J. Shiu,* U.-S. Jeng, Y.-S. Huang, Y.-H. Lai, H.-F. Lu, C.-T. Liang,*; I.-J. Hsu, C.-H. Su, C. Su,* I. Chao, A.-C. Su, S.-H. Lin* Global and Local Structural Changes of Cytochrome c and Lysozyme Characterized by a Multigroup Unfolding Process. BIOPHYSICAL JOURNAL 2008, 94, 4828-4836.
- Y.-C. Chang, Y.-D. Chen, C.-H. Chen, Y.-S. Wen, J. T. Lin*, Y.-Y. Chen, M.-Y. Kuo, I. Chao* Crystal Engineering for π−π Stacking via Interaction between Electron-Rich and Electron-Deficient Heteroaromatics. JOURNAL OF ORGANIC CHEMISTRY 2008, 94,4828-4836.
- H.-F. Lu,* F.-Y. Li,* C.-C. Lin, K. Nagaya, I. Chao, S. H. Lin The Fragmentation of Ethanol Cation under an Electric Field: An ab initio/RRKM Study. CHEMICAL PHYSICS LETTERS 2007, 443, 178-182.
- F. Valiyev, W.-S. Hu, H.-Y. Chen, M.-Y. Kuo, I. Chao,* Y.-T. Tao* Synthesis and Characterization of Anthra[2,3-b]thiophene and Naphtha[2,3-b]thiophenes for Organic Field Effect Transistor Applications. CHEMISTRY OF MATERIALS 2007, 19, 3018-3026.
- M.-F. Wu, S.-J. Yeh, C.-T. Chen,* H. Murayama, T. Tsuboi,* W.-S. Li, I. Chao,* S.-W. Liu, J.-K. Wang* A Quest of High-Performance Host Materials for Electrophosphorescence Blue Dopant. ADVANCED FUNCTIONAL MATERIALS 2007, 17, 1887-1895.
- M.-Y. Kuo, H.-Y. Chen, I. Chao* Cyanation: Providing Three-in-One Advantage for the Design of n-Type Organic Field-Effect Transistors. CHEMISTRY-A EUROPEAN JOURNAL 2007, 13, 4750-4758.
- Chen HY and Chao I Toward the rational design of functionalized pentacenes: Reduction of the impact of functionalization on the reorganization energy. CHEMPHYSCHEM 2006-09, 7(9), 2003-2007.
- Chu, JH, Li, WS, Chao, I, Lee, GH and Chung, WS Regioselectivity in the 1,3-dipolar cycloaddition of adamantylidenefulvene and its modification by inclusion in cyclodextrins' solutions. TETRAHEDRON 2006-07, 62(31), 7380-7389.
- Yen, ML, Li, WS, Lai, CC, Chao, I and Chiu, SH Dual-action acid/base- and base/acid-controllable molecular switch. ORGANIC LETTERS 2006-07, 8(15), 3223-3226.
- Cheng, PN, Huang, PY, Li, WS, Ueng, SH, Hung, WC, Liu, YH, Lai, CC, Wang, Y, Peng, SM, Chao, I and Chiu, SH Is [N+-H center dot center dot center dot O] hydrogen bonding the most important noncovalent interaction in macrocycle-dibenzylammonium ion complexes?. JOURNAL OF ORGANIC CHEMISTRY 2006-03, 71(6), 2373-2383.
- S.-J. Lo, W.-S. Li, Y.-H. Chen and I. Chao* Theoretical study of remote control of hydrogen bond strengths in donor-bridge-acceptor systems: Principles for designing effective bridges with substituent tuning.. CHEMISTRY-A EUROPEAN JOURNAL 2005, 11, 6533-6542.
- H.-Y. Chen and I. Chao* Effect of perfluorination on the charge-transport properties of organic semiconductors: Density functional theory study of perfluorinated pentacene and sexithiophene.. Chem. Phys. Lett. 2005, 410, 539-545.
- H.-Y. Chen* and I. Chao* Ionization-induced proton transfer in model DNA base pair: A theoretical study of the radical ions of the 7-azaindole dimer.. ChemPhysChem. 2004, 5, 1855-1863.
- H.-N. Lee, T.-M. Su and I. Chao* Rotamer dynamics of substituted simple alkanes. 1. A classical trajectory study of collisional orientation and alignment of 1-bromo-2-chloroethane with argon.. J. Phys. Chem. A 2004, 108, 2567-2575.
- J.-H. Chu, W.-S. Li, I. Chao* and W.-S. Chung* Face selectivity in the reactions of 2,4-disubstituted adamantanes and their modification by inclusion in F-cyclodextrin solutions.. Tetrahedron 2004, 60, 9493-9501.
- T.-S. Hwang, N. Juan, H.-Y. Chen, C.-C. Chen, S.-J. Lo and I. Chao* Control of hydrogen bond strengths through push-pull effects triggered by a remote reaction center: A theoreticals study.. CHEMISTRY-A EUROPEAN JOURNAL 2004, 10, 1616-1624.
- Li, W.-S., Chung, W.-S. and Chao I.* A computational study of regioselectivity in a cyclodextrin mediated Diels-Alder reaction: Revelation of site selectivity and the importance of shallow binding and multiple binding modes.. CHEMISTRY-A EUROPEAN JOURNAL 2003, 9, 951-962.
- G. H. Hakimelahi*, P.-C. Li, A. A. Moosavi-Movahedi, J. Chamani, G. A. Khodarahmi, T. W. Ly, F. Valiyev, M. K. Leong, S. Hakimelahi, K.-S. Shia and I. Chao* Application of barton photochemical reaction in the synthesis of 1-dethia-3-aza-1-carba-2-oxacephem: A novel agent against resistant pathogenic microorganisms.. ORGANIC & BIOMOLECULAR CHEMISTRY 2003, 1, 2461-2467.
- Leung, M.-k.;* Mandal, A. B.; Wang, C.-C.; Lee, G.-H.; Peng, S.-M.;* Cheng, H.-L.; Her, G.-R.; Chao, I.;* Lu, H.-f.; Sun, Y.-C.; Shiao, M.-Y.; Chou, P.-T.* Self-Complementarity of Oligo-??-aminopyridines: A New Class of Hydrogen-Bonded Ladders. J. Am. Chem. Soc. 2002, 124, 4287-4297.
- Li, P.-C.; Wang, T.-S.; Lee, G.-H.; Liu, Y.-H.; Wang, Y.;* Chen, C.-T.;* Chao, I.* Theoretical Study and X-Ray Determination of Bianthrones: Long C-C Bond Length and Preferred Gauche Conformation. J. Org. Chem. 2002, 67, 8002-8009.
- Chao, I.;* Chen, K.-W.; Hwang, T.-S.; Liu, K.-T.* Density Functional Theory Calculations of 17O and 13C NMR Chemical Shifts for Aromatic Acyl Chlorides. J. Phys. Org. Chem. 2001, 14, 591-596.
- Chao, I.;* Hwang, T.-S. Remote Communication between Charge Centers and Hydrogen-Bonding Sites: Possibility for a Signal Transducer?. Angew. Chem. Int. Ed. 2001, 40, 2703-2705.
- Chao, I.;* Shih, J. H.; Wu, H.-J.* Quantum Mechanical Study on the Facial Selectivity of Dioxa and Trioxa Cage Molecules. J. Org. Chem. 2000, 65, 7523-7533.
- Chou, T.-s.; Chen, H.-C.; Yang, W.-C.; Li, W.-S.; Chao, I.; Lee, S.-J.* Type-Two Intramolecular Diels-Alder Reactions of Pyrazolo-o-quinodimethanes. J. Org. Chem. 2000, 65, 5760-5767.
- Hwang, M.-J.;* Chu, P.-Y.; Chen, J.-C.; Chao, I. Conformational Analysis of Three Pyrophosphate Model Species: Diphosphate, Methyl Diphosphate, and Triphosphate. J. Comput. Chem. 1999, 20, 1702-1715.
- Kan, L.-s.;* Lin, W.-C.; Yadav, R. D.; Shih, J. H.; Chao, I. NMR Studies of the Tautomerism in Pseudoisocytidine. Nucleosides Nucleotides 1999, 18,1091-1093.
- Lin, H. C.;* Lin, Y. S.; Chao, C. Y.; Chao, I.; Li, T.-W. Hydrogen-Bonded Liquid Crystalline Polymers with Various Angular Pendant Groups. Macromolecules, 1998, 31, 7298-7311.
- Li, T.-W.; Chao, I.;* Tao, Y.-T. The Relationship between Packing Structures and Head Groups of Self-Assembled Monolayers on Au(111): Bridging Experimental Observations through Computer Simulations. J. Phys. Chem. B 1998, 102, 2935-2946.
- Chao, I.;* Chen, K.-W.; Liu, K.-T.;* Tsoo, C.-C. Density Functional Theory Calculations of 17O NMR Chemical Shifts of Substituted ??,??,??-Trifluoromethyl Aryl Ketones. Tetrahedron Lett. 1998, 39, 1001-1004.
- Chang, Y.-P.; Su, T.-M.;* Li, T.-W.; Chao, I.* Intramolecular Hydrogen Bonding, Gauche Interactions, and Thermodynamic Functions of 1,2-Ethanediamine, 1,2-Ethanediol, and 2-Aminoethanol: a Global Conformational Analysis. J. Phys. Chem. A 1997, 101, 6107-6117.
- Chao, I.;* Lu, H.-f.; Chou, T.-s. A Theoretical Study of the Exceptional Thermal Reactivity of 2-Phenyloxazolo-3-sulfolene: Roles Played by Aromaticity and Strain. J. Org. Chem. 1997, 62, 7882-7884.
- Lee, S.-J.;* Tzeng, C.-B.; Lin, S.-C.; Chao, I.; Lu, H.-F.; Chou, T.-s. Novel Crossed Diels-Alder Reactions of 1-(2-Butadienyl)pyridinium Bromide. J. Org. Chem. 1996, 61, 9293-9296.
- Liu, K.-T.;* Tsao, M.-L.; Chao, I. Variation of Coplanarity between Aryl Ring and Cationic Center at Transition State. Agreement of Results of Solvolytic Study with ab initio Calculations. Tetrahedron Lett. 1996, 37, 4173-4176.
- Chao, I.;* Chen, J.-C. Resolving the Puzzling Eclipsed Conformation of the Methyl Group in a Tricyclic Orthoamide Trihydrate. Angew. Chem. Int. Ed. Engl. 1996, 35, 195-197.
- Sheu, J.-T.; Lin, C.-C.; Chao, I.; Wang, C.-C.; Peng, S.-M.* Linear Trinuclear Three-Centered Metal-Metal Multiple Bonds: Synthesis and Crystal Structure of [M3(dpa)4Cl2] (M=RuII & RhII, dpa=bis(2-pyridyl)amido anion). J. Chem. Soc., Chem. Commun. 1996, 315-316.
- Garcia-Garibay, M. A.; Shin, S. H.; Chao, I.; Houk, K. N.; Khan, S. I. Solid-State 13C CPMAS NMR and Molecular Mechanics Study of Conformational Recognition in Mixed Crystals of Two Phenylalkyl Ketones. Chem. Mater. 1994, 6, 1297-1306.
- Yeh, T.-S.; Chang, Y.-P.; Su, T.-M.;* Chao, I.* Global Conformational Analysis of 1,2-Ethanediol. J. Phys. Chem. 1994, 98, 8921-8929..
- Lee, S.-J.;* Chien, C.-J.; Peng, C.-J.; Chao, I.; Chou, T.-s.* 1-(2-Butadienyl)-pyridinium Bromide: A Novel Diene in Diels-Alder Reactions. J. Org. Chem. 1994, 16, 4367-4369.
- Chang, S.-C.; Chao, I.; Tao, Y.-T.* Structures of Self-Assembled Monolayers of Aromatic-Derivatized Thiols on Evaporated Gold and Silver Surfaces: Implication on Packing Mechanism. J. Am. Chem. Soc. 1994, 15, 6792-6805.
- Chao, I.* Molecular Mechanics. CHEMISTRY (THE CHINESE CHEM. SOC., TAIWAN CHINA) 1994, 52, 180-185.
- Chao, I.; Diederich, F. Catalytic Cyclophanes. VII. Esterase Activity of a Bisimidazolyl-Cyclophane. Recl. Trav. Chim. Pays-Bas 1993, 112(6), 335-338.
- Diederich, F.; Smithrud, D. B.; Sanford, E. M.; Wyman, T. B.; Ferguson, S. B.; Carcanague, D. R.; Chao, I.; Houk, K. N. Solvent Effects in Molecular Recognition. Acta Chem. Scand. 1992, 46, 205-215.
- Jorgensen, W. L.; Nguyen, T. B.; Sanford, E. M.; Chao, I.; Houk, K. N.; Diederich, F. Enhanced View of Structure and Binding for Cyclophane-Arene Complexes through Joint Theoretical and Experimental Study. J. Am. Chem. Soc. 1992, 114, 4003-4004.
- Deshayes, K.; Broene, R. D.; Chao, I.; Knobler, C. B.; Diederich, F. Synthesis of the Helicopodands : Novel Shapes for Chiral Clefts. J. Org. Chem. 1991, 56, 6787-6795.
- Ettl, R.; Chao, I.; Diederich, F.; Whetten, R. L. Isolation of C76, a Chiral (D2) Allotrope of Carbon. Nature 1991, 353, 149-153.
- Diederich, F.; Whetten, R. L.; Thilgen, C.; Ettl, R.; Chao, I.; Alvarez, M. M. Fullerene Isomerism : Isolation of C2v-C78 and D3-C78. Science 1991, 254, 1768-1770.
- Smithrud, D. B.; Sanford, E. M.; Chao, I.; Ferguson, S. B.; Carcanague, D. R.; Evanseck, J. D.; Houk, K. N.; Diederich, F. Solvent Effects in Molecular Recognition. Pure and Applied Chemistry 1990, 62, 2227-2236.
- Diederich, F.; Sch?mann, G.; Chao, I. Designed Water-Soluble Macrocyclic Esterases: From Nonproductive to Productive Binding. J. Org. Chem. 1988, 53, 2744-2757.
- Yang, M.-H.; Liang, J.-C.; Chao, I.; Liu, S.-L. Synthesis and Properties of Trimethylphenoxy- and Tetramethylphenoxysilanes. J. Chin. Chem. Soc. 1985, 32, 341-347.
- 趙奕姼,2008,〈通往綠色化學的任意門〉,《科學月刊》,第39卷第2期,頁122-125。

