中央研究院化學研究所-學術研究
研究漸凍症中蛋白質凝聚的新工具:用光控分子探針調節細胞內蛋白質的凝聚體
Breakthrough in ALS Research: Light-Activated Molecular Tool Controls Protein Behavior in Cells
Nat. Commun. 2024, 15, 5686.
Hao-Yu Chuang, Ruei-Yu He, Yung-An Huang, Wan-Ting Hsu, Ya-Jen Cheng, Zheng-Rong Guo, Niaz Wali, Ing-Shouh Hwang, Jiun-Jie Shie & Joseph Jen-Tse Huang *
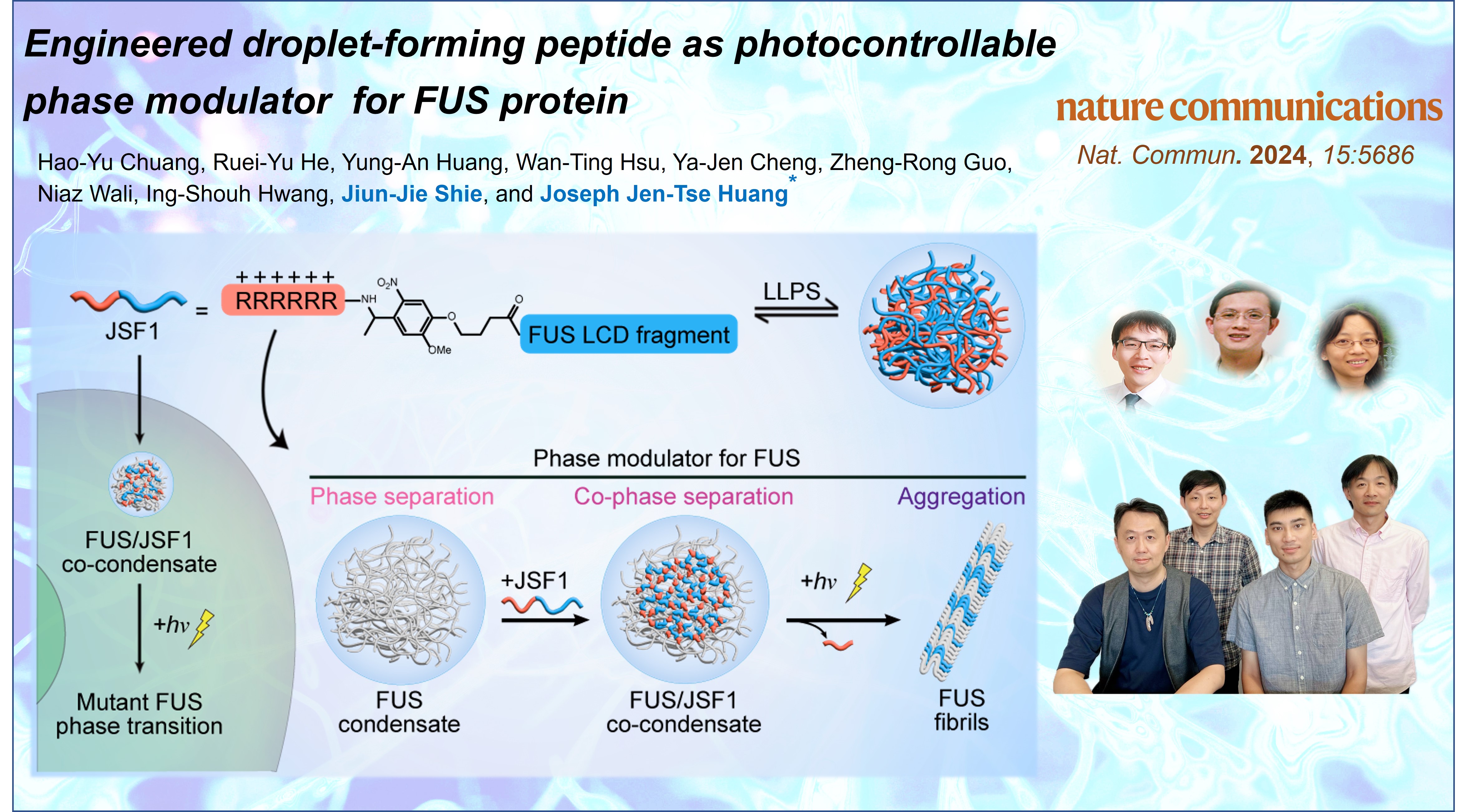
在細胞內生物分子的凝聚體(condensates)是很小的液滴,對於調節體內的化學反應扮演著重要的功能。如果這些液滴的性質發生改變,可能會導致疾病。例如,會形成凝聚體的FUS蛋白就被發現和漸凍症(ALS)有關,因此了解細胞內的凝聚現象是一個重要的議題。然而,目前缺乏有效的工具來研究或是控制這些凝聚體。為了解決這個問題,中研院化學所的研究團隊發明了一種新的工具,能夠利用光來控制與漸凍症相關的蛋白質凝聚現象,為漸凍症的研究帶來了新的方向。
黃人則博士和他的團隊於近期創造了一種突破性的光控分子探針。該探針除了本身就能夠形成凝聚體外,也可以利用光照調控FUS蛋白凝聚體的流動性,讓研究人員能夠進一步觀察和控制這些蛋白質在細胞內的變化。結合光控探針與光學影像平台,團隊也發現了FUS蛋白的凝聚體在細胞質中的流動性對神經細胞的存活至關重要。此一發現也為開發漸凍症以及其他神經退化性疾病的治療方法提供了全新的思路。該實驗室長期致力於漸凍症的相關研究,他們也希望這項成果能夠增加大眾對於漸凍症相關研究的認識及支持。
這項研究由黃人則博士的團隊完成,莊皓宇博士生是第一作者,並與物理所的黃英碩博士和化學所的謝俊結博士合作。他們的發現於2024年7月6日發表在《自然通訊》(Nature Communications)期刊上。
期刊聯結:https://www.nature.com/articles/s41467-024-50025-5
Understanding how proteins behave inside our cells is crucial for advancing treatments for diseases like amyotrophic lateral sclerosis (ALS). Scientists at the Institute of Chemistry, Academia Sinica, have developed an innovative molecular tool that uses light to control the behavior of proteins associated with ALS, offering new hope for treatment.
Biomolecular condensates are tiny droplets within cells that play a key role in regulating chemical reactions. When these droplets don’t form or behave correctly, it can lead to diseases. For instance, the protein FUS forms condensates, and its malfunction is linked to ALS. Until now, scientists lacked effective tools to study and manipulate these condensates.
Dr. Joseph Jen-Tse Huang and his team have created a groundbreaking photocontrollable molecular probe that can change the state of FUS protein condensates from liquid to solid with light exposure. This allows researchers to observe and control how these proteins behave inside cells. Using this light-activated probe, the team discovered that the fluidity of FUS protein condensates in the cytoplasm is crucial for the health of neuronal cells. This insight opens up new possibilities for developing ALS treatments and understanding other motor neuron diseases.
Dr. Huang’s team, including first author Hao-Yu Chuang from TIGP-CBMB at Academia Sinica, collaborated with experts from the Institute of Physics and the Institute of Chemistry. Their findings, published on July 6, 2024, in Nature Communications, mark a significant advancement in the field. “We believe this molecular tool will not only help in ALS research but also in understanding and treating other neurodegenerative diseases,” said Dr. Huang. The team’s dedication to ALS research over the years highlights the importance of this development.
Article link:https://www.nature.com/articles/s41467-024-50025-5
