Institute of Chemistry, Academia Sinica – Research
讓「壞蛋白」被分手!雙極性胜肽分子 抑制亨丁頓氏舞蹈症的新策略
Nanoscopic insights of amphiphilic peptide against Huntington’s disease
Adv. Sci.. 2020, 7, 1901165. (Online published: Dec. 09, 2019; Journal published: Jan. 22, 2020)
Ruei-Yu He,* Xiang-Me Lai, Chia-Sui Sun, Te-Shien Kung, Jhu-Ying Hong,
Yu-Song Jheng, Wei-Neng Liao, Jen-Kun Chen, Yung-Feng Liao, Pang-Hsien Tu,*
and Joseph Jen-Tse Huang*
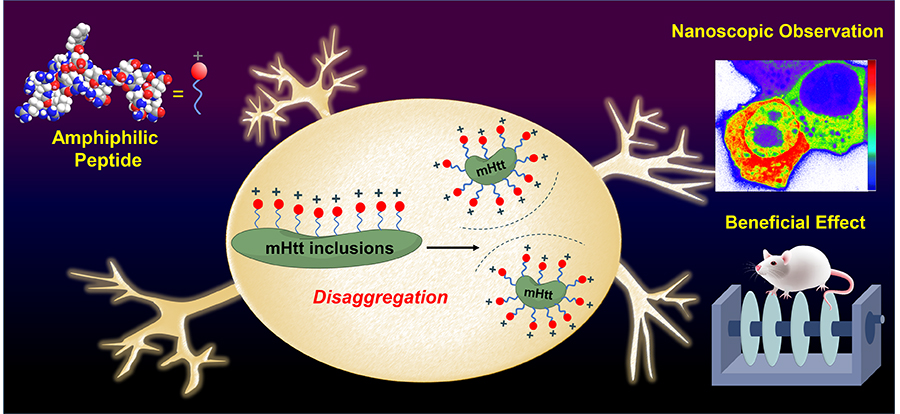
Figure 1. The amphiphilic peptide can perturb the oligomer assembly of mHtt through the charge repulsion, ameliorate mHtt-induced neurological damage, and rescue the memory deficit
雙極性胜肽分子,一端可以辨識變異的亨丁頓蛋白,另一端帶正電,能利用電荷排斥力,阻止變異的亨丁頓蛋白(mHtt)堆積,改善變異蛋白所造成的運動與認知功能失調。
With the increase in the aging population, an effective therapeutic regimen is still an unmet medical need for different neurodegenerative disorders. Dr. Joseph Jen-Tse Huang, an associate research fellow at the Institute of Chemistry in Academia Sinica, Dr. Pang-Hsien Tu, a former assistant research fellow at the Institute of Biomedical Science, and their research teams recently report a self-assembled amphiphilic peptide that can inhibit protein aggregation implicated in the pathogenesis of Huntington’s disease (HD). This new finding was published in Advanced Science on January, 2020.
Current literature indicates the oligomerization and aggregation of the mutant Huntingtin (mHtt) protein are closely related to HD proteinopathy. However, for the difficulties in observing the dynamic aggregation and oligomerization process of mHtt in vivo, the evaluation of potential drugs is usually restricted. By combing lifetime-based fluorescence microscopies and biophysical tools, Dr. Huang and Dr. Tu demonstrate the designed amphiphilic peptide, which targets the mHtt at an early stage, can perturb the oligomer assembly process nanoscopically, suppress the amyloid property of mHtt, and ameliorate mHtt-induced neurological damage in cell and mouse models.
Dr. Huang remarked, “While tracking the transient and subtle changes in protein conformational dynamics in vitro is feasible, observing the aforementioned phenomenon in live cells remains challenging. By establishing a combined biophotonic platform, we are the first to visualize not only the aggregation but also the oligomerization process perturbed by the amphiphilic peptide in live neuronal cells in real time.” Moreover, they found the amphiphilic peptide is able to transport to the brain and rescue the memory deficit through intranasal administration. The related patent in this cooperative and multidiscipline study has just been approved in Taiwan and under application in other country. This study provides new insight into the design of a targeted therapeutic agent and creates a biophotonic platform for monitoring the oligomerization process in neurodegenerative diseases.
Dr. Ruei‐Yu He is the first author in this study. The corresponding authors include Dr. Joseph Jen-Tse Huang, Dr. Ruei‐Yu He, and Dr. Pang-Hsien Tu. Dr. Huang also appreciates the contributions from Dr. Jen-Kun Chen and Dr. Yung-Feng Liao from the Institute of Biomedical Engineering and Nanomedicine in National Health Research Institutes and the Institute of Cellular and Organismic Biology in Academia Sinica.
The full article entitled “Nanoscopic Insights of Amphiphilic Peptide against the Oligomer Assembly Process to Treat Huntington’s Disease” can be now found at the Advanced Science website at: https://onlinelibrary.wiley.com/doi/full/10.1002/advs.201901165
Media Contact:
Dr. Joseph Jen-Tse Huang, Associated Research Fellow, Institute of Chemistry, Academia Sinica
(Tel) +886-2-5572-8652, jthuang@gate.sinica.edu.tw
Ms. Pei-Chun Kuo, Media Team, Secretariat, Central Administrative Office, Academia Sinica
(Tel) +886-2-2789-8821, deartree@gate.sinica.edu.tw
Mr. Chang-Hung Chen, Media Team, Secretariat, Central Administrative Office, Academia Sinica
(Tel) +886-2-2789-8059, changhung@gate.sinica.edu.tw
亨丁頓氏舞蹈症(Huntington’s Disease)是一種神經退化疾病,因為突變基因讓亨丁頓蛋白(mHtt)異常聚集,造成腦部神經持續性退化。患者發病後手腳不聽使喚,像在跳舞一樣,末期急速退化,平均存活約15至20年,目前仍無法完全治癒。
中央研究院化學研究所黃人則副研究員與前生醫所杜邦憲助研究員組成的研究團隊,設計出「雙極性胜肽分子」,能夠將一團團聚集的亨丁頓蛋白分散開來,將此分子透過鼻腔給藥至罹病小鼠後,小鼠的運動與認知功能失調明顯得到改善。本研究已於今(2020)年1月刊登於《先進科學》(Advanced Science)。
雙極性胜肽分子可辨識變異的「壞蛋白」使其分散開來
黃人則指出,罹患亨丁頓氏舞蹈症的病人,變異的亨丁頓蛋白帶有過長的聚麩醯胺酸鏈(polyglutamine),隨著聚麩醯胺酸鏈的增加,亨丁頓蛋白容易堆積在腦部,破壞神經元。研究團隊設計出雙極性胜肽分子,分子的其中一端可以辨識變異的亨丁頓蛋白並附著上去,分子的另一端帶正電,可以利用電荷排斥力,把聚集的變異蛋白分散開來,讓變異的亨丁頓壞蛋白「被分手」,減少對神經細胞的毒性及損傷。
研究團隊也發現,將雙極性胜肽分子透過鼻腔給藥至罹病小鼠,可以使小鼠在滾筒跑步機上維持平衡,運動功能獲得顯著改善,並且在T型迷宮實驗中,展現較好的認知功能。
率先從活細胞追蹤變異蛋白的動態結構變化
過去有關亨丁頓氏舞蹈症的研究,不易從生物體內觀察變異蛋白聚集化的過程,黃人則表示,本研究另一突破是建立生物光電影像平台,透過螢光影像顯微系統,觀測亨丁頓蛋白在活細胞中聚集與分散的過程。研究團隊在小鼠神經細胞實驗中,把變異的亨丁頓蛋白標示上兩種不同的螢光蛋白,此時,若變異的亨丁頓蛋白互相靠近,將改變螢光蛋白的放光特性,藉此觀察變異蛋白是否聚集,以及雙極性胜肽分子的抑制效果。目前已獲得和此研究相關的臺灣專利,其他國家專利則正申請中。
本論文由本院以及科技部支持。第一作者為本院化學所何瑞玉博士、通訊作者為黃人則副研究員、何瑞玉博士,及前生醫所杜邦憲助研究員。研究團隊還包括國衛院生醫工程與奈米醫學所的陳仁焜副研究員,以及本院細胞與個體生物學研究所的廖永豐副研究員。
期刊論文連結: https://onlinelibrary.wiley.com/doi/full/10.1002/advs.201901165
新聞聯繫人:
黃人則副研究員,中央研究院化學研究所
(Tel) 02-5572-8652,jthuang@gate.sinica.edu.tw
郭姵君,中央研究院秘書處媒體小組
(Tel) 02-2789-8821,deartree@gate.sinica.edu.tw
陳昶宏,中央研究院秘書處媒體小組
(Tel) 02-2789-8059,changhung@gate.sinica.edu.tw