Institute of Chemistry, Academia Sinica – Research
難以捉摸的三配位雙陽離子氫化硼錯合物
The Elusive Three-Coordinate Dicationic Hydrido Boron ComplexJ. Am. Chem. Soc. 2014, 136, 914-917. Wen-Ching Chen, Ching-Yu Lee, Bo-Chao Lin, Yu-Chen Hsu, Jiun-Shian Shen, Chao-Ping Hsu*, Glenn P. A. Yap, and Tiow-Gan Ong*
具有難以捉摸的電子組態及獨特鍵結環境之硼物種,一直是研究分子主族化學的一個核心主題。累積過去研究具有陽離子特性之硼物種所得的知識,使其潛在的應用範圍更加廣泛,其中包括聚合反應、烯烴類的硼氫化反應及硼烷氨的脫氫化反應等。
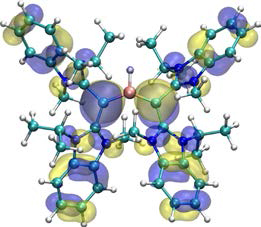
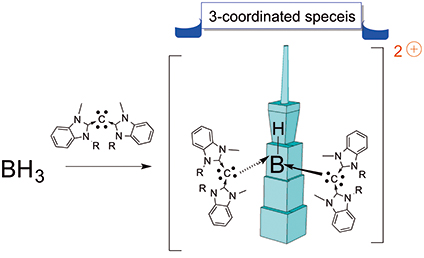
在本篇論文中,我們描述了一個具有氫化物配位基的三配位雙陽離子硼烷的合成,這是前所未有的硼化物結構。有趣的是不需使用高親電性的路易士酸作為前驅物,即可利用配位基”同碳雙碳烯” (carbodicarbene) 與硼烷進行反應得之。由於同碳雙碳烯與氮異環碳烯 (NHC) 在鍵結參數上的微妙差異,使其形成高電荷性之主族元素的錯合物,可以透過電荷離域與共振結合,並從第二電子對額外進行p對稱性贈與,使結構具有相對的穩定性。
雖然這個偶然的發現,目前只能激起實驗室的好奇心,但是這些結果將鋪設一條未來研究高度親電性路易士酸化學應用於有機合成及催化反應的康莊大道。
The search for unique bonding environments of boron species featuring elusive electronic configurations has been a central theme in molecular main group chemistry. Such fundamental investigations on cationic boron species have led to an accumulation of knowledge for a broad range of potential applications including polymerization, hydroboration of alkenes and dehydrogenation of ammonia-borane.
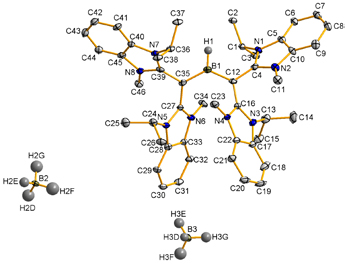
In here, we have described the formation of a hitherto unknown three-coordinate dicationic borane bearing a hydride ligand. Interestingly, supporting ligand carbodicarbene gave unprecedented reaction with BH3 without using more highly electrophilic Lewis acid precursors. The subtle variation in bonding parameters of carbodicarbene ligands vs NHC’s demonstrated that the stability of highly charged main group complexes could be accessed through charge delocalization with resonance conjugation and additional p-symmetry donation from second electron pairs.
Although this serendipitous finding can currently be regarded as a laboratory curiosity, these results pave the way for future studies in highly electrophilic Lewis acid chemistry with interesting potential applications in organic synthesis and catalysis.