Institute of Chemistry, Academia Sinica – Research
(6,6) 碳奈米環帶的十二重去芳香化酯化反應
Twelvefold Dearomative Esterification of (6,6)Carbon NanobeltAngew. Chem. Int. Ed. 2025, e202510544
Tsubasa Okumura, Daiki Imoto, Yuri Arachi, Akiko Yagi, Takehisa Maekawa* and Kenichiro Itami*
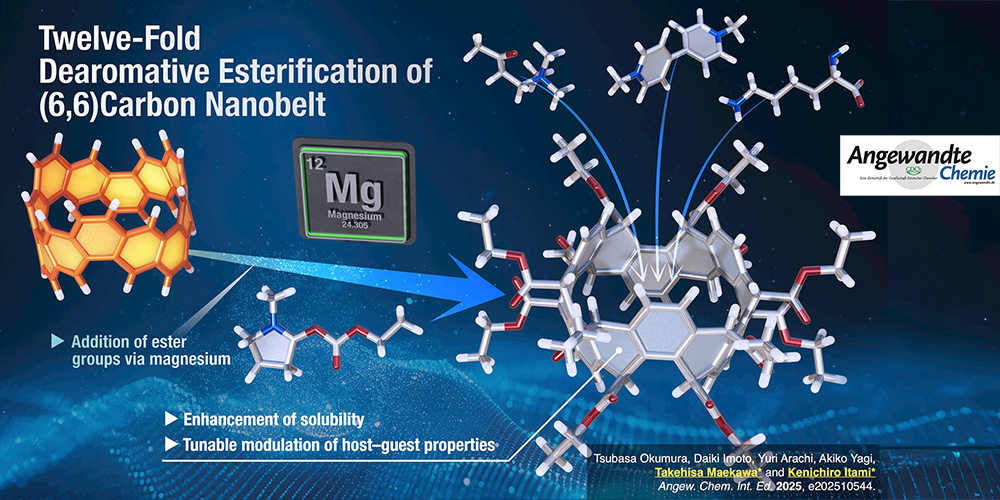
Carbon nanobelts (CNBs), cyclic aromatic hydrocarbons with belt-shaped topologies, have growing interest as structurally unique and optoelectronically promising nanocarbons. Yet, strategies for their direct and site-selective functionalization remain scarce. An international research team at the Institute of Chemistry, Academia Sinica, reported a magnesium-mediated, multifold dearomative esterification of (6,6)CNB, enabling the installation of twelve ester groups in a single transformation. Single-crystal X-ray structural analysis revealed regioselective dearomatization patterns, whereas mechanistic studies uncovered a radical-driven pathway involving oxyiminium intermediate generated in situ from alkyl chloroformates and amide solvents. Computational analysis further indicated that strain release in the CNB framework drives the high degree of functionalization. This methodology not only expands the synthetic toolbox for aromatic nanobelts but also allows tunable modulation of their host–guest properties via enhanced conformational flexibility.
碳奈米環帶 (Carbon nanobelts, CNBs) 是一類具有環帶狀拓撲結構的環狀芳香族碳氫化合物,因其獨特的結構與具潛力的光電特性,日益受到關注。然而,目前對於其直接且位點選擇性的官能基化策略仍相當有限。本所的研究團隊近期報導一項由鎂介導的多重去芳香化酯化反應,可在一次轉化中於 (6,6)CNB 上安裝多達十二個酯基。單晶X光繞射結構分析顯示其具區域選擇性的去芳香化模式;而機制研究則揭示該反應經由自由基驅動的路徑進行,反應中產生的氧亞胺中間體由烷基氯甲酸酯與醯胺溶劑原位生成。計算模擬分析進一步指出,CNB骨架中的應變釋放為其高度官能基化提供驅動力。此一方法不僅拓展了芳香環帶類奈米碳材料的合成工具箱,亦可藉由提升構型柔性來調控其主–客體性質,實現奈米碳材料的可調式功能化。
