Institute of Chemistry, Academia Sinica – Research
Angew. Chem., Int. Ed. 2019, 58, 16775 –16779. Chun-Wei Chang, Chia-Hui Wu, Mei-Huei Lin, Pin-Hsuan Liao, Chun-Chi Chang, Hsiao-Han Chuang, Su-Ching Lin, Sarah Lam, Ved Prakash Verma, Chao-Ping Hsu*, Cheng-Chung Wang*
醣類分子在生物化學、藥物化學的產、學中均扮演非常重要的角色,然而受限於研究素材的取得不易,大大的侷限了研究的發展。雖然近幾年來有機合成策略提供了一個可能的解決方向,但在醣鏈結反應的開發過程中,仍然缺乏有效的準則來克服立體位向選擇性問題,此合成困境除大幅降低合成效率外,更導致醣類分子的開發極其緩慢。數十年來,雖然有大量的研究和論文發表,此一立體位向之控制仍舊是一個近乎隨機且聽天由命的過程。為克服此合成困境,本所王正中與許昭萍老師實驗室攜手合作,藉由有機合成及數值分析建立了一個新穎、簡易且方便的參數系統,此參數亦可提供AI系統作為演算的依據。未來全自動的醣類分子合成將可望成為常態。我們相信此一計畫帶來的成果能為醣化學和有機化學學界及產業界帶來革命性的影響和衝擊。此研究成果著重於醣予體(glycosyl donor)之量化以及大數據分析,藉由數值量化提供一個新的準則來解釋並預測複雜的醣鏈結反應,此結果跳脫傳統有機化學的泥淖,因而得到高影響係數 (Impact factor) 國際期刊的認同,已於近期發表於Angew. Chem., Int. Ed. 2019, 58, 16775 –16779。
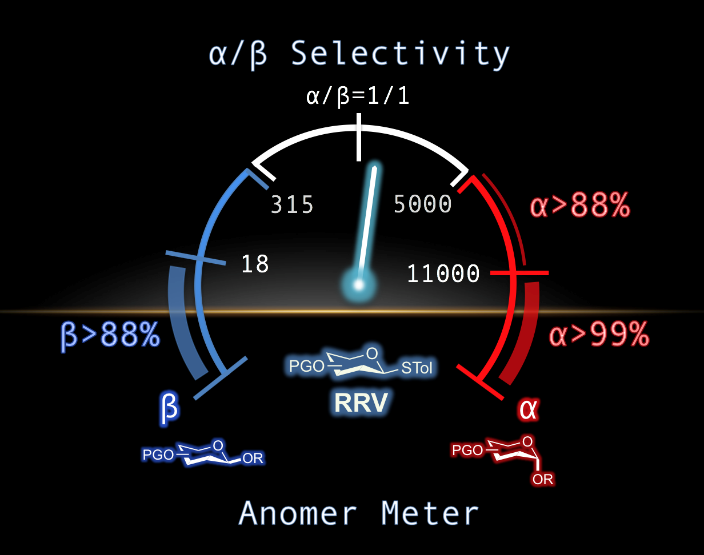
Stereocontrolled chemical glycosylation remains a major challenge despite vast efforts reported over many decades and so far still mainly relies on trial and error. Now it is shown that the relative reactivity value (RRV) of thioglycosides is an indicator for revealing stereoselectivities according to four types of acceptors. Mechanistic studies show that the reaction is dominated by two distinct intermediates: glycosyl triflates and glycosyl halides from N-halosuccinimide (NXS)/TfOH. The formation of glycosyl halide is highly correlated with the production of α-glycoside. These findings enable glycosylation reactions to be foreseen by using RRVs as an α/β-selectivity indicator and guidelines and rules to be developed for stereocontrolled glycosylation.