Institute of Chemistry, Academia Sinica – Research
解密醣類合成之活化劑效應以及離子交換行為
Unraveling the promoter effect and the roles of counterion exchange in glycosylation reactionySci. Adv. 2023, 9(42), eadk0531.
Chang, C.-W.; Lin, M.-H.; Chiang, T.-Y.; Wu, C.-H.; Lin T.-C.;
Wang, C.-C*
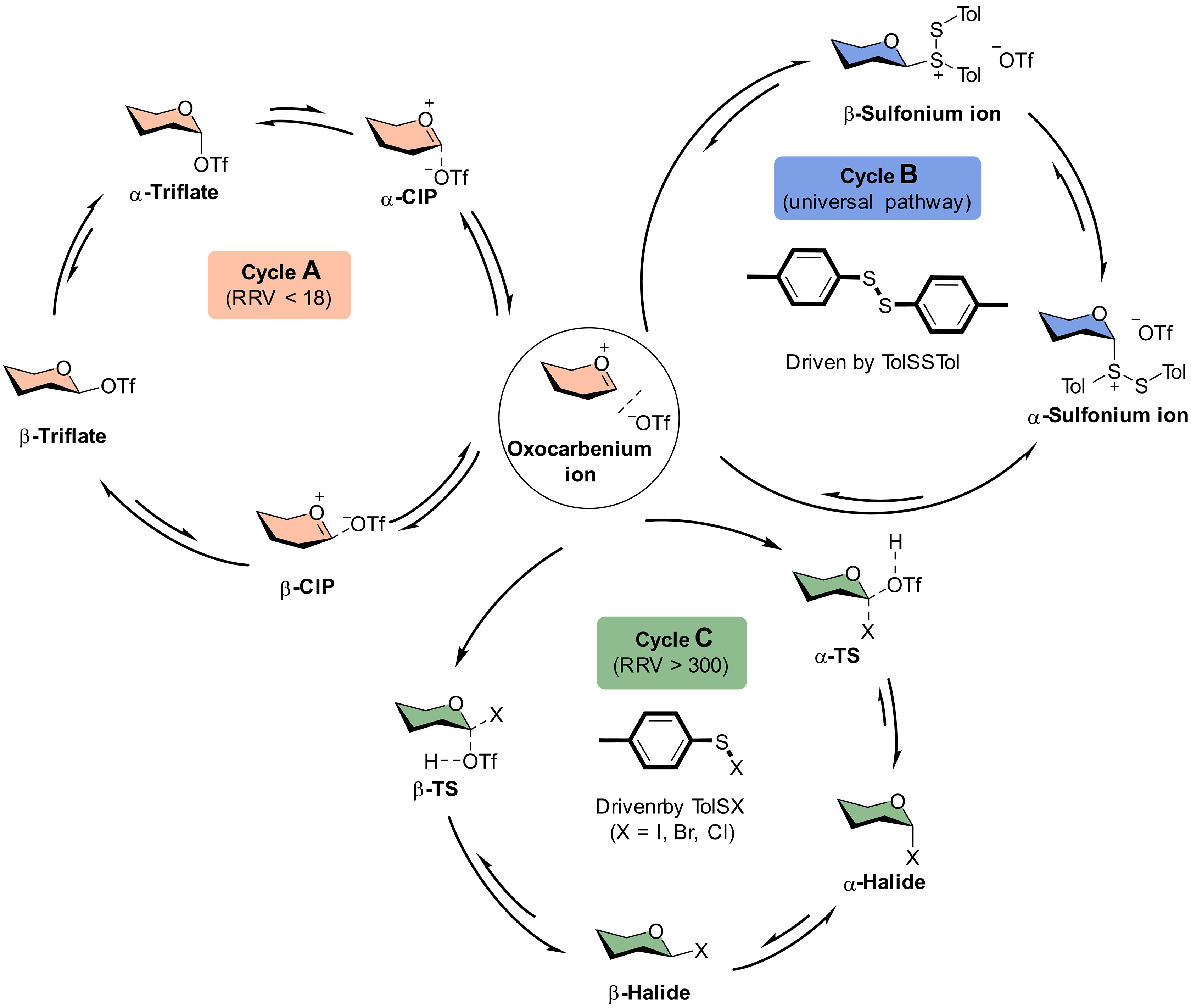
Chemical synthesis is one of the most reliable methods to access high-purity homogeneous oligosaccharides and their derivatives in large quantities. However, achieving high stereoselectivity in glycosidic bond formation remains a significant challenge in carbohydrate synthesis. This challenge arises from a limited understanding of the reaction mechanism, which is an area that has not been thoroughly explored and clarified. In this study, we conducted an extensive investigation into the glycosylation mechanism, employing low-temperature Nuclear Magnetic Resonance (NMR) and statistical methodologies. Our research led to the discovery of a novel pathway driven by counterion exchanges and reaction byproducts, which elucidates the contributions of intermediates to stereochemistry. Additionally, we introduced relative reactivity values (RRVs), acceptor nucleophilic constants (Aka), and Hammett substituent constants (σ values) as comprehensive indicators of mechanistic pathways. These findings have the potential to significantly simplify the optimization of building blocks and reaction conditions in carbohydrate synthesis.
化學合成是取得高純度寡醣及其衍生物的最可靠的方法之一。然而,有效建構高立體選擇性醣甘鍵仍為現今合成的一項重要挑戰。此窘境源於化學家對反應機制的了解不夠純熟,且反應中間體之行為尚未被有效探討和澄清。敝實驗室於本次發表中成功透過低溫核磁共振(NMR)捕獲醣基中間體,並進一步地結合統計分析對其行為作廣泛的分析。我們的研究首度證實中間體離子交換的可能性,且機制途徑更可透過相對反應活性值(RRV)、受體親核常數(Aka)和 Hammett 取代基常數(σ 值)作為的綜合指標來達到預測及控制的效果。此研究成果有效提升醣類合成效率並進一步的簡化合成中組件(building blocks)的篩選。。
