:::

Research Fellow (2020-);Associate Research Fellow (2013-2020);Assistant Research Fellow (2005-2013);B.S., National Changhua University of Education (1993);Ph.D., Indiana University at Bloomington, U.S.A. (2001);Postdoctoral Scientist, U.S. DOE Argonne National Laboratory (2001-2004);Postdoctoral Fellow, National Tsing Hua University (2004-2005) ;

Research Interests
The research in Dr. Chiang’s group is focused on the rational synthesis of novel transition metal catalysts for efficient hydrogen production. Design of the catalyst is inspired by the active site of [FeFe] hydrogenase in virtue of its superior ability for hydrogen production. The active site consists of a {Fe2S2} core attached by a [4Fe4S] cluster. Two major issues essential to catalysis of the active site of [FeFe] hydrogenase are addressed: the substrate accessible site and the attached [4Fe4S] unit.
Molecular manipulation techniques are deployed to construct structural models with variations to simulate structural confinement by the protein pocket. The products under reducing and acidic conditions are isolated for the purpose of mechanistic elucidation. Aside from the influence of the peptide chains in the vicinity of the active site, the rotated geometry of the distal Fe center within the active site is also induced by changes of electronic structures of the Fe2 core. The [4Fe4S] unit within the H cluster plays a key role on the electronic changes. Several Fe-S model complexes with redox-active units are characterized to gain insights of the electronic influence by the presence of the redox-active fragments.
The hydrogenase metalloenzyme and the artificial bio-inspired catalysts for hydrogen production
Discovering a new type of green energy resource of low cost is of contemporary importance to ease rapid growth of global demand for fossil fuels that produces excessive amount of CO2 and causes tremendous climate changes. Molecular hydrogen is considered as the next generation clean fuel, since its stored chemical energy releases with formation of water. In biological systems, hydrogenases are used to catalyze H2. Understanding the catalytic mechanism thus facilitates chemists to design artificial biomimitic catalysts to hydrogen production.The research in Dr. Chiang’s group is focused on the rational synthesis of novel transition metal catalysts for efficient hydrogen production. Design of the catalyst is inspired by the active site of [FeFe] hydrogenase in virtue of its superior ability for hydrogen production. The active site consists of a {Fe2S2} core attached by a [4Fe4S] cluster. Two major issues essential to catalysis of the active site of [FeFe] hydrogenase are addressed: the substrate accessible site and the attached [4Fe4S] unit.
Molecular manipulation techniques are deployed to construct structural models with variations to simulate structural confinement by the protein pocket. The products under reducing and acidic conditions are isolated for the purpose of mechanistic elucidation. Aside from the influence of the peptide chains in the vicinity of the active site, the rotated geometry of the distal Fe center within the active site is also induced by changes of electronic structures of the Fe2 core. The [4Fe4S] unit within the H cluster plays a key role on the electronic changes. Several Fe-S model complexes with redox-active units are characterized to gain insights of the electronic influence by the presence of the redox-active fragments.
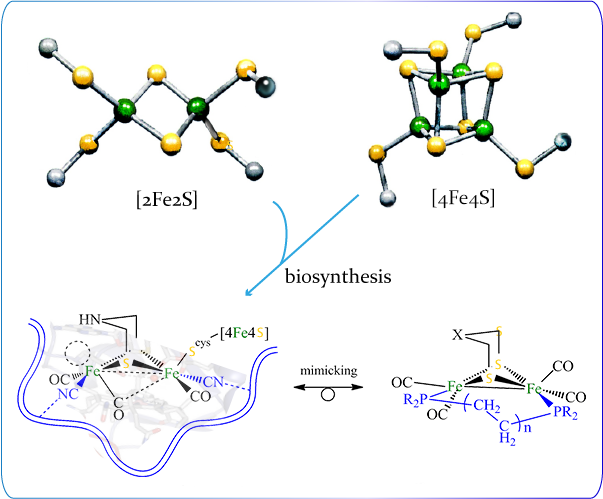
Mimicking the active site of [FeFe] hydrogenase
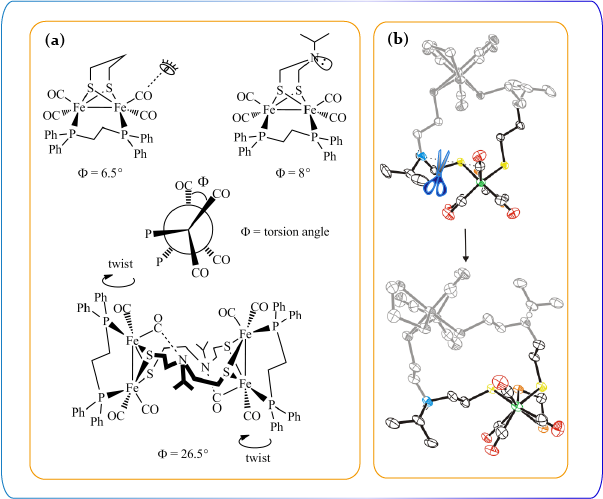
The influeneces of the primary (a) and secondary (b) ligand fields
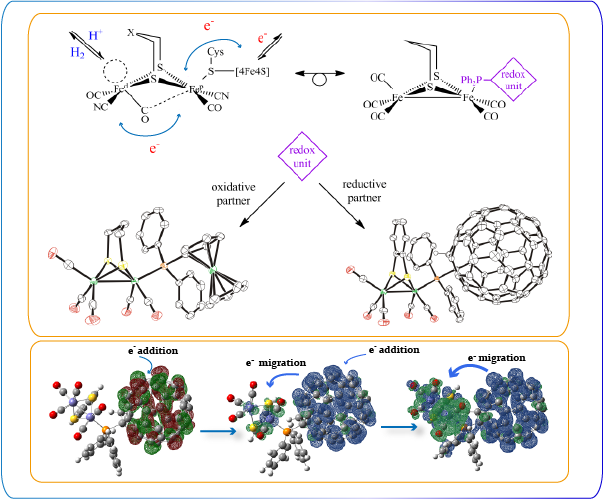
Electronic influence of the redox partners
The dioxygenase metalloenzyme and the artificial bio-inspired catalysts for oxidation of aromatic carbons
The catabolism of catechol species including halogenated derivatives in bacterial degradative pathways is achieved by ring-cleaving dioxygenases. The specific metalloenzymes utilize molecular oxygen to generate the aliphatic products, which allows recycling of the carbons sequestered in aromatic organics. Two categories for oxygen insertion to the aromatic carbons are classified on the basis of the position of the C-C bond: the intradiol and extradiol cleavage. The former type breaks the 1,2-carbon-carbon bond, resulting in cis,cis-muconic acids. The latter cleavages the 2,3-carbon-carbon bond to produce muconic semialdehydes. The Fe ion is contained in the active site of both types of dioxygenases. For the extradiol dioxygenase, Mn2+ (Mn-MndD, Arthrobacter globiformis) is also identified. Here, Dr. Chiang’s group is interested in activation of molecular oxygen via dioxygenases to generate oxidation products of the aromatic compounds. The research focus is on the mechanistic study to obtain advanced knowledge related to how bacteria utilize aromatic carbons in term of energy and decontamination of halogenated molecules.
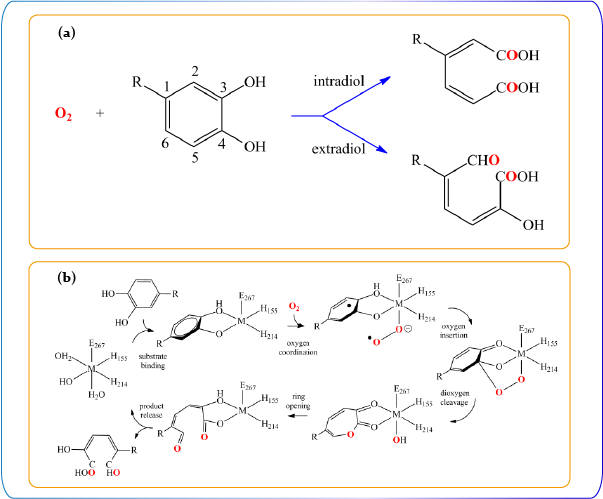
(a) Ring cleavage by catechol dioxygenase (b) Proposed catalytic cycle of homoprotocatechuate 2,3-dioxygenase
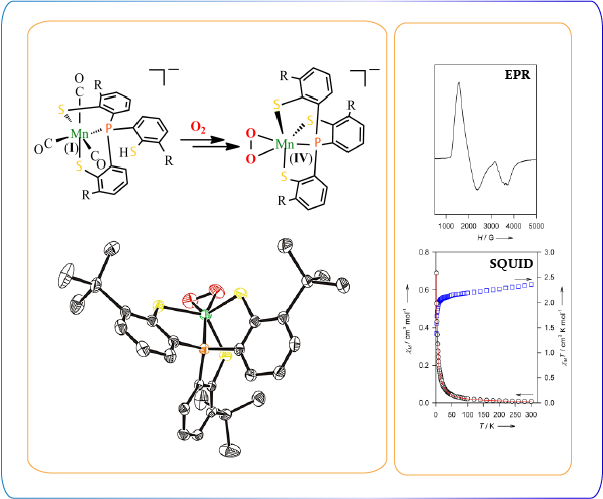
Synthesis and characterization of the peroxomanganese(IV) complex
Nano-sized porous catalysts
In search of potential applications of artificial catalysts for pilot runs, Dr. Chiang is developing nano-sized porous catalysts. Nanomaterials are of several advantages: They can serve as a support to catalytically active transition metals. Catalytic selectivity and efficiency are tunable by pore orifices and effective surface areas. Dr. Chiang has teamed up with several research groups to synthesize metal-organic coordination polymers from a variety of transition metal ions and organic ligands based on a combinatorial strategy. Of particular interest is the fact that when catalytic metal sites are immobilized on the backbones of three-dimensional structures, magnetic behaviors are observed due to interactions among the paramagnetic metal centers. The magnetic property in turn may influence the catalysis. Such networks furnished with paramagnetic ions are attractive in terms of potential magneto applications.
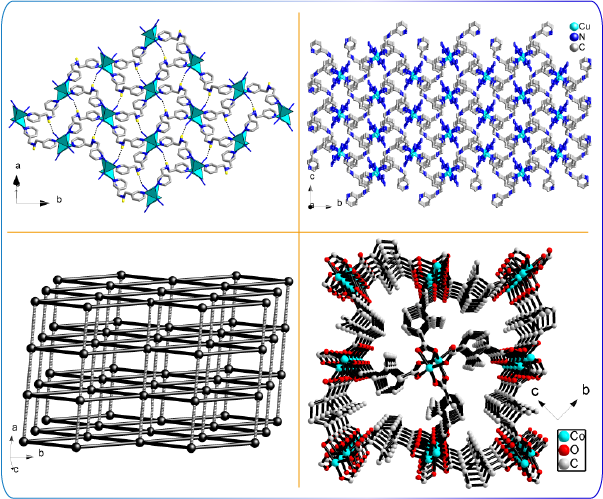
Nano-porous structures
Awards & Honors
- "傑出青年學者獎" (2019-09)
Selected Publications
- Inamdar, A. I.; Kamal, S.; Usman, M.; Chiang, M.-H.*; Lu, K.-L.* Design strategies for dielectric metal–organic frameworks and their applications in microelectronic devices. Coord. Chem. Rev. 2024, 502, 215596.
- Kang, H.-W.; Liu, Y.-C.; Shao, W.-K.; Wei, Y.-C.; Hsieh, C.-T.; Chen, B.-H.; Lu, C.-H.; Yang, S.-D.; Cheng, M.-J.; Chou, P.-T.*; Chiang, M.-H.*; Wu, Y.-T.* Synthesis, structural analysis, and properties of highly twisted alkenes 13,13’-bis(dibenzo[a,i]fluorenylidene) and its derivatives. Nat. Commun. 2023, 14, 5248.
- Paste, R.; Li, S.; Fu, J.-H.; Chiang, Y.-H.; Inamdar, A. I.; Chiang, M.-H.; Tung, V.*; Lin, H.-C.*; Chu, C. W.* Capillary-induced self-crumpled and sulfur-deficient MoS2 nanosheets inhibit polysulfide cycling in lithium–sulfur batteries. J. Mater. Chem. A 2023, 11, 8265-8276.
- Chen, B.-H.; Xu, J.-J.; Lai, W.-R.; Chang, C.-K.; Chen, J.-L.; Lee, J.-F.; Chen, J.-M.; Sheu, H.-S.; Lee, J.-J.; Kubota, Y.; Chiang, M.-H.; Kitagawa, Y.*; Chuang, Y.-C.*; Hsu, I -J.* Structure determination and magnetic studies of triazole chelated Co(II) coordination polymers. J. Chin. Chem. Soc. 2023, 70, 1187-1199.
- Lai, T. Y.; Chen, C.-T.; Chu, K.-T.; Chien, S.-Y.; Ong, T.-G.*; Chiang, M.-H.* Biologically inspired 3Fe4S cluster as structural mimics of FeMoco M-cluster. J. Chin. Chem. Soc. 2023, 70, 1029-1037.
- Hsieh, C.-C.; Liao, P.-K.; Chen, C.-W.; Chiang, M.-H.; Horng, Y.-C.* The effect of anions in the synthesis and structure of pyrazolylamidino copper(II) complexes. Dalton Trans. 2023, 52, 4429-4441.
- Hsieh, C.-C.; Li, C.-Y.; Chiang, M.-H.; Horng, Y.-C.* Catalytic O2 activation toward oxidative N–S bond formation by a thiolato Fe(iii) complex. Chem. Commun. 2022, 58, 12943-12946.
- Chiang, C.-K.; Liu, Y.-C.; Chu, K.-T.; Chen, J.-T.; Tsai, C.-Y.; Lee, G.-H.; Chiang, M.-H.*; Lee, C.-M.* Stable Bimetallic FeII/{Fe(NO)2}9 Moiety Derived from Reductive Transformations of a Diferrous-dinitrosyl Species. Inorg. Chem. 2022, 61, 16325-16332.
- Jadhav, T. S.; Abbas, S. A.; Chu, K.-T.; Wu, W.-T.; Hsu, Y.-Y.; Lee, G.-H.; Chien, S.-Y.; Chu, C.-W.*; Chiang, M.-H.* Surficial grafting of organoimido moieties enhances the capacity performance of oxometallic clusters. Dalton Trans. 2022, 51, 14875-14881.
- Wu, Y.-C.; Liu, Y.-C.; Tsai, S.-W.; Chu, K.-T.; Chen, H.-J.; Wu, C.-Y.; Hsu, Y.-Y.; Hsieh, C.-C.; Liu, W.-J.; Kong, K. V.*; Chiang, M.-H.* Demethylation of an artificial hydrogenase agent for prolonged CO release and enhanced anti-tau aggregation activity, Chem. Commun. 2022, 58, 7245-7248. (featured as a cover paper)
- Cheng, M.-C.; Lee, G.-H.; Lin, T.-S.; Liu, Y.-C.; Chiang, M.-H.; Chen C.-h.*; Peng, S.-M.* Metal replacement in the syntheses of MAMBMC heterometallic metal-string complexes: MPdM'(dpa)4Cl2. J. Chin. Chem. Soc. 2022, 69, 1438-1448.
- Martinez, B.; Chang, D.-N.; Huang, Y.-C.; Dong, C.-L.; Chiu, T.-W.*; Chiang, M.-H.*; Kuo, C.-H.* Formation of a p-n heterojunction photocatalyst by the interfacing of graphitic carbon nitride and delafossite CuGaO2. J. Chin. Chem. Soc. 2022, 69, 1042-1050.
- Inamdar, A. I.; Sainbileg, B.; Lin, C.-J.; Usman, M.; Kamal, S.; Chiou, K.-R.; Pathak, A.; Luo, T.-T.; Bayikadi, K. S.; Sankar, R.; Chen, J.-W.; Tseng, T.-W.; Chen, R.-S.*; Hayashi, M.*; Chiang, M.-H.*; Lu, K.-L.* Regimented Charge Transport Phenomena in Semiconductive Self-Assembled Rhenium Nanotubes. ACS Appl. Mater. Interfaces 2022, 14, 12423-12433.
- Sheelam, A.; Balu, S.; Muneeb, A.; Bayikadi, K. S.; Namasivayam, D.; Siddharthan, E. E; Inamdar, A. I; Thapa, R.; Chiang, M.-H.; Huang, S.-J. I.; Sankar, R.* Improved Oxygen Redox Activity by High-Valent Fe and Co3+ Sites in the Perovskite LaNi1–xFe0.5xCo0.5xO3. ACS Appl. Energy Mater. 2022, 5, 343-354.
- Xu, J.-X.; Jiang, Y.-S.; Chen, C.-H.; Sathishkumar, N.; Chu, K.-T.; Chiang, M.-H.; Chen, H.-T.; Han, J.-L.* Enantioselective Organocatalytic Three-Component Vinylogous Michael/Aldol Tandem Reaction among 3-Alkylidene oxindoles, Methyleneindolinones, and Aldehydes. J. Org. Chem. 2022, 87, 197-210.
- Chen, P.-Y.; Liu, Y.-C.; Hung, H.-Y.; Pan, M.-L.; Wei, Y.-C.; Kuo, T.-C.; Cheng, M.-J.; Chou, P.-T.*; Chiang, M.-H.*; Wu, Y.-T.* Diindeno[2,1-b:2′,1′-h]biphenylenes: Syntheses, Structural Analyses, and Properties. Org. Lett. 2021, 23, 8794-8798.
- Cheng, M.-C.; Cheng, C.-H.; Chen, P.-J.; Lin, T.-S.; Lee, G.-H.; Liu, Y.-C.; Chiang, M.-H.; Peng, S.-M.*; Helical homometallic trinickel string complexes with mixed hard nitrogen and sulfur donors: structural and magnetic studies. Bull. Chem. Soc. Jpn. 2021, 94, 2092-2099.
- Lin, Z.-Y.; Datta, A.; Das, K.; Inamdar, A. I.; Hsieh, H.-Y.; Huang, Y.-H.; Chiang, M.-H.; Liu, C.-J.; Chen, L.-R.; Lin, Y.-W.; Lee, H. M.* Proton-Conducting Cobalt(II) 3D MOFs Incorporating Bis(imidazole) and Polycarboxylate Linkages: Framework Topology and Interpenetration, Cryst. Growth Des. 2021, 21, 5594-5602.
- Inamdar, A. I.; Kaisar, N.; Kamal, S.; Luo, T.-T.; Jou, S.; Chu, C.-W.*; Chiang, M.-H.;* Lu Lu, K.-L.* Design of a Metal–Organic Framework-Derived Co9S8/S Material for Achieving High Durability and High Performance of Lithium–Sulfur Batteries. ChemElectroChem 2021, 8, 3040-3048. (featured as a cover paper)
- Silalahi, R. P.; Chiu, T.-H.; Kao, J.-H.; Wu, C.-Y.; Yin, C.-W.; Liu, Y.-C.; Chen, Y. J.*; Saillard, J.-Y.; Chiang, M.-H.*; Liu, C.-W.* Synthesis and Luminescence Properties of Two-Electron Bimetallic Cu-Ag and Cu-Au Nanoclusters via Copper Hydride Precursors. Inorg. Chem. 2021, 60, 10799-10807.
- Inamdar, A. I.; Sainbileg, B.; Kamal, S.; Bayikadi, K. S.; Sankar, R.; Luo, T. T.; Hayashi, M.*; Chiang, M.-H.*; Lu, K.-L.* Water-assisted spin-flop antiferromagnetic behaviour of hydrophobic Cu-based metal–organic frameworks, Dalton Trans. 2021, 50, 5754-5758. (featured as a cover paper)
- Xu, Jing-Xiang; Chu, K.-T.; Chiang, M.-H.; Han, J.-L.* Organocatalytic asymmetric allylic alkylation of 2-methyl-3-nitroindoles: a route to direct enantioselective functionalization of indole C(sp3)–H bonds. Org. Biomol. Chem. 2021, 19, 1503-1507.
- Chiang, M.-H.*; Pelmenschikov, V.*; Gee, L. B.; Liu, Y.-C.; Hsieh, C.-C.; Wang, H.; Yoda, Y.; Matsuura, H.; Li, L.; Cramer, S. P.* High-Frequency Fe–H and Fe–H2 Modes in a trans-Fe(η2-H2)(H) Complex: A Speed Record for Nuclear Resonance Vibrational Spectroscopy. Inorg. Chem. 2021, 60, 555-559.
- Jadhav, T. S.; Abbas, S. A.; Liu, Y.-C.; Wu, W.-T.; Lee, G.-H.; Chih-Wei Chu,* Ming-Hsi Chiang* Discrete Metal-Oxide Clusters with Organofunctionalization as High-Performance Anode Materials. ACS Appl. Energy Mater. 2021, 4, 643-654.
- Cheng, M.-C.; Lee, G.-H.; Lin, T.-S.; Liu, Y.-C.; Chiang, M.-H.; Peng, S.-M.* A new series of heteronuclear metal strings, MRhRh(dpa)4Cl2 and MRhRhM(dpa)4X2, from the reactions of Rh2(dpa)4 with metal ions of group 7 to group 12. Dalton Trans. 2021, 50, 520-534.
- Gee, L. B.; Pelmenschikov, V.*; Wang, H.; Mishra, N.; Liu, Y.-C.; Yoda, Y.; Tamasaku, K.; Chiang, M.-H.*; Cramer, S. P* Vibrational characterization of a diiron bridging hydride complex–a model for hydrogen catalysis. Chem. Sci. 2020, 11, 5487-5493.
- Cheng, M.-C.; Huang, R.-X.; Liu, Y.-C.; Chiang, M.-H.; Lee, G.-H.; Song, Y.; Lin, T.-S.; Peng S.-M.* Structures and paramagnetism of five heterometallic pentanuclear metal strings containing as many as four different metals: NiPtCo2Pd(tpda)4Cl2. Dalton Trans. 2020, 49, 7299-7303.
- Chiang, C.-K.; Chu, K.-T.; Lin, C.-C.; Xie, S.-R.; Liu, Y.-C.; Demeshko, S.; Lee, G.-H.; Meyer, F.; Tsai, M.-L.; Chiang, M.-H.*; Lee, C.-M.* Photoinduced NO and HNO Production from Mononuclear {FeNO}6 Complex Bearing a Pendant Thiol. J. Am. Chem. Soc. 2020, 142, 8649-8661.
- Inamdar, A. I.; Pathak, A.; Usman, M.; Chiou, K.-R.; Tsai, P.-H.; Mendiratta, S.; Kamal, S.; Liu, Y.-H.; Chen, J.-W.; Chiang, M.-H.; Lu, K.-L.* Highly Hydrophobic Metal–Organic Framework for Self-Protecting Gate Dielectrics. J. Mater. Chem. A 2020, 8, 11958-11965.
- Kuo, Y.-L.; Tseng, C.-Y.; Tseng, C.-W.; Chu, K.-T.; Liu, Y.-C.; Chiang, M.-H.; Saeki, A.; Tao, Y.-T.*; Chen, H.-H.* Polymerization of Columnar Mesogens Tethered with Diacetylenic Side Chains. ACS Appl. Polym. Mater. 2020, 2, 248-255.
- Barik, S. K.; Chiu, T.-H.; Liu, Y.-C.; Chiang, M.-H.*; Gam, F.; Chantrenne, I.; Kahlal, S.; Saillard, J.-Y.*; Liu, C. W.* Mono- and hexa-palladium doped silver nanoclusters stabilized by dithiolates. Nanoscale 2019, 11, 14581-14586.
- Wu, J.-Y.*; Yuan, P.-T.; Hsiao, C.-C.; Chang, H.-K.; Liu, Y.-C.; Hsu, L.-J.; Chiang, M.-H.* Structural diversity in polymeric and discrete complexes constructed by divalent transition metals and unsymmetrical quasi semirigid pyridinecarboxylate isomers. J. Solid St. Chem. 2019, 277, 701-712.
- Wu, W.-Y.; Hsu, C.-N.; Hsieh, C.-H.; Chiou, T.-W.*; Tsai, M.-L.*; Chiang, M.-H.; Liaw, W.-F.* NO-to-[N2O2]2–-to-N2O Conversion Triggered by {Fe(NO)2}10-{Fe(NO)2}9 Dinuclear Dinitrosyl Iron Complex. Inorg. Chem. 2019, 58, 9586-9591.
- Boominathan, S. S. K.; Chang, K.-H.; Liu, Y.-C.; Wang, C.-S.; Wu, C.-F.; Chiang, M.-H.*; Chou, P.-T.*, Wu, Y.-T.* Diindeno‐fused Dibenzo[a,h]anthracene and Dibenzo[c,l]chrysene: Syntheses, Structural Analyses and Properties. Chem. Eur. J. 2019, 25, 7280-7284.
- Lee, C.-M.*; Sankaralingam, M.; Chuo, C.-H.; Tseng, T.-H.; Chen, P. P.-Y.*; Chiang, M.-H.*; Li, X. X.; Lee, Y. M.; Nam, W.* A Mn(IV)–peroxo complex in the reactions with proton donors. Dalton Trans. 2019, 48, 5203-5213.
- Chakrahari, K. K.; Silalahi, R. P. B.; Wang, X.; Kahlal, S.; Liu, Y.-C.; Chiang, M.-H.; Saillard, J.-Y.; Liu, C. W.* Synthesis of Bimetallic Copper-Rich Nanoclusters Encapsulating a Linear Palladium Dihydride Unit. Angew. Chem. Ed. Int. 2019, 58, 4943-4947.
- Hsieh, C.-C.; Liu, Y.-C.; Tseng, M.-C.; Chiang, M.-H.; Horng, Y.-C.* Dioxygen activation by a dinuclear thiolate-ligated Fe(II) complex. Dalton Trans. 2019, 48, 379-386.
- Wong, G. F.; Yeung, L. F.; Tsoi, H. Y.; Chan, H.-S.; Chiang, M.-H.; Lee, H. K.* A Mononuclear Iron(II) Bis(guanidinate) Complex: Synthesis, Structure, and Reactivity. Eur. J. Inorg. Chem. 2019, 98-109.
Update: 2024-01-12
Journal Paper
- Yu-Chiao Liu, Kai-Ti Chu, Hong-Ru Wang, Gene-Hsiang Lee, Mei-Chun Tseng, Cheng-Hsin Wang, Yih-Chern Horng,* Ming-Hsi Chiang* Chloride- and Hydrosulfide-Bound 2Fe Complexes as Models of the Oxygen-Stable State of [FeFe] Hydrogenase. ANGEWANDTE CHEMIE-INTERNATIONAL EDITION 2024-07, 63, e202408142.
- Arif I. Inamdar, Saqib Kamal, Muhammad Usman, Ming-Hsi Chiang*, Kuang-Lieh Lu* Design strategies for dielectric metal–organic frameworks and their applications in microelectronic devices. COORDINATION CHEMISTRY REVIEWS 2024-03, 502, 215596.
- Hao-Wen Kang, Yu-Chiao Liu, Wei-Kai Shao, Yu-Chen Wei, Chi-Tien Hsieh, Bo-Han Chen, Chih-Hsuan Lu, Shang-Da Yang, Mu-Jeng Cheng, Pi-Tai Chou*, Ming-Hsi Chiang*, Yao-Ting Wu* Synthesis, structural analysis, and properties of highly twisted alkenes 13,13’-bis(dibenzo[a,i]fluorenylidene) and its derivatives. NATURE COMMUNICATIONS 2023-08, 14, 5248.
- Ting Yi Lai, Chang-Ting Chen, Kai-Ti Chu, Su-Ying Chien, Tiow-Gan Ong*, Ming-Hsi Chiang* Biologically inspired 3Fe4S cluster as structural mimics of FeMoco M-cluster. JOURNAL OF THE CHINESE CHEMICAL SOCIETY 2023-05, 70, 1029-1037.
- Bo-Hau Chen, Jun-Jia Xu, Wei-Ren Lai, Chung-Kai Chang, Jeng-Lung Chen, Jyh-Fu Lee, Jin-Ming Chen, Hwo-Shuenn Sheu, Jey-Jau Lee, Yoshiki Kubota, Ming-Hsi Chiang, Yasutaka Kitagawa*, Yu-Chun Chuang*, I-Jui Hsu* Structure determination and magnetic studies of triazole chelated Co(II) coordination polymers. JOURNAL OF THE CHINESE CHEMICAL SOCIETY 2023-05, 70, 1187-1199.
- Rohan Paste, Shenghan Li, Jui-Han Fu, Yu-Hsiang Chiang, Arif I. Inamdar, Ming-Hsi Chiang, Vincent Tung,* Hong-Cheu Lin*, Chih Wei Chu* Capillary-induced self-crumpled and sulfur-deficient MoS2 nanosheets inhibit polysulfide cycling in lithium–sulfur batteries. JOURNAL OF MATERIALS CHEMISTRY A 2023-04, 11, 8265-8276.
- Chang-Chih Hsieh, Po-Kuang Liao, Chia-Wei Chen, Ming-Hsi Chiang, Yih-Chern Horng* The effect of anions in the synthesis and structure of pyrazolylamidino copper(II) complexes. DALTON TRANSACTIONS 2023-01, 52, 4429-4441.
- Chang-Chih Hsieh, Cheng-Yao Li, Ming-Hsi Chiang, Yih-Chern Horng* Catalytic O2 activation toward oxidative N–S bond formation by a thiolato Fe(iii) complex. CHEMICAL COMMUNICATIONS 2022-11, 58, 12943-12946.
- Chuan-Kuei Chiang, Yu-Chiao Liu, Kai-Ti Chu, Jing-Ting Chen, Cheng-Yeh Tsai, Gene-Hsiang Lee, Ming-Hsi Chiang*, Chien-Ming Lee* Stable Bimetallic FeII/{Fe(NO)2}9 Moiety Derived from Reductive Transformations of a Diferrous-dinitrosyl Species. INORGANIC CHEMISTRY 2022-10, 61, 16325-16332.
- Tushar Sanjay Jadhav, Syed Ali Abbas, Kai-Ti Chu, Wen-Ti Wu, Yu-Yi Hsu, Gene- Hsiang Lee, Su-Ying Chien, Chih-Wei Chu,* Ming-Hsi Chiang* Surficial grafting of organoimido moieties enhances the capacity performance of oxometallic clusters. DALTON TRANSACTIONS 2022-08, 51, 14875-14881.
- Yun-Chin Wu, Yu-Chiao Liu, Shu-Wei Tsai, Kai-Ti Chu, Hsin-Jou Chen, Cheng-Yun Wu, Yu-Yi Hsu, Chang-Chih Hsieh, Wang-Jing Liu, Kien Voon Kong*, Ming-Hsi Chiang* (featured as a cover paper) Demethylation of an artificial hydrogenase agent for prolonged CO release and enhanced anti-tau aggregation activity. CHEMICAL COMMUNICATIONS 2022-06, 58, 7245-7248.
- Benjamin Martinez, Dai-Ning Chang, Yu-Cheng Huang, Chung-Li Dong, Te-Wei Chiu*, Ming-Hsi Chiang*, Chun-Hong Kuo* Formation of a p-n heterojunction photocatalyst by the interfacing of graphitic carbon nitride and delafossite CuGaO2. JOURNAL OF THE CHINESE CHEMICAL SOCIETY 2022-05, 69, 1042-1050.
- Ming-Chuan Cheng, Gene-Hsiang Lee, Tien-Sung Lin, Yu-Chiao Liu, Ming-Hsi Chiang, Chun-hsien Chen*, Shie-Ming Peng* Metal replacement in the syntheses of MAMBMC heterometallic metal-string complexes: MPdM'(dpa)4Cl2. JOURNAL OF THE CHINESE CHEMICAL SOCIETY 2022-05, 69, 1438-1448.
- Arif I. Inamdar, Batjargal Sainbileg, Chi-Jia Lin, Muhammad Usman, Saqib Kamal, Kuan-Ru Chiou, Abhishek Pathak, Tzuoo-Tsair Luo, Khasim Saheb Bayikadi, Raman Sankar, Jenq-Wei Chen, Tien-Wen Tseng, Ruei-San Chen*, Michitoshi Hayashi*, Ming-Hsi Chiang*, Kuang-Lieh Lu* Regimented Charge Transport Phenomena in Semiconductive Self-Assembled Rhenium Nanotubes. ACS APPLIED MATERIALS & INTERFACES 2022-03, 14, 12423-12433.
- Jing-Xiang Xu, Yi-Syun Jiang, Chih-Hao Chen, Nadaraj Sathishkumar, Kai-Ti Chu, Ming-Hsi Chiang, Hsin-Tsung Chen, Jeng-Liang Han* Enantioselective Organocatalytic Three-Component Vinylogous Michael/Aldol Tandem Reaction among 3-Alkylidene oxindoles, Methyleneindolinones, and Aldehydes. JOURNAL OF ORGANIC CHEMISTRY 2022-01, 87, 197-210.
- Anjaiah Sheelam, Sakthipriya Balu, Adil Muneeb, Khasim Saheb Bayikadi, Dhenadhayalan Namasivayam, Erakulan E Siddharthan, Arif I Inamdar, Ranjit Thapa, Ming-Hsi Chiang, Song-Jeng Isaac Huang, Raman Sankar* Improved Oxygen Redox Activity by High-Valent Fe and Co3+ Sites in the Perovskite LaNi1–xFe0.5xCo0.5xO3. ACS Applied Energy Materials 2022-01, 5, 343-354.
- Pei-Yun Chen, Yu-Chiao Liu, Hui-Yu Hung, Ming-Lun Pan, Yu-Chen Wei, Tung-Chun Kuo, Mu-Jeng Cheng, Pi-Tai Chou*, Ming-Hsi Chiang*, Yao-Ting Wu* Diindeno[2,1-b:2′,1′-h]biphenylenes: Syntheses, Structural Analyses, and Properties. ORGANIC LETTERS 2021-10, 23, 8794-8798.
- Cheng, Ming-Chuan; Cheng, Chien-Hung; Chen, Po-Jung; Lin, Tien-Sung; Lee, Gene-Hsiang; Liu, Yu-Chiao; Chiang, Ming-Hsi; Peng, Shie-Ming* Helical homometallic trinickel string complexes with mixed hard nitrogen and sulfur donors: structural and magnetic studies. Bulletin of the Chemical Society of Japan 2021-09, 94, 2092-2099.
- Zih-Yi Lin, Amitabha Datta, Kuheli Das, Arif I. Inamdar, Hsi-Yuan Hsieh, Yao-Huei Huang, Ming-Hsi Chiang, Chia-Jyi Liu, Liang-Rui Chen, Yang-Wei Lin, Hon Man Lee* Proton-Conducting Cobalt(II) 3D MOFs Incorporating Bis(imidazole) and Polycarboxylate Linkages: Framework Topology and Interpenetration. CRYSTAL GROWTH & DESIGN 2021-08, 21, 5594-5602.
- Arif I. Inamdar, Nahid Kaisar, Saqib Kamal, Tzuoo-Tsair Luo, Shyankay Jou, Chih-Wei Chu,* Ming-Hsi Chiang,* Kuang-Lieh Lu* (featured as a cover paper) Design of a Metal–Organic Framework-Derived Co9S8/S Material for Achieving High Durability and High Performance of Lithium–Sulfur Batteries. ChemElectroChem 2021-07, 8, 3040-3048.
- Silalahi, Rhone P.; Chiu, Tzu-Hao; Kao, Jhen-Heng; Wu, Chun-Yen; Yin, Chi-Wei; Liu, Yu-Chiao; Chen, Yuan Jang;* Saillard, Jean-Yves; Chiang, Ming-Hsi;* Liu, Chen-Wei* Synthesis and Luminescence Properties of Two-Electron Bimetallic Cu-Ag and Cu-Au Nanoclusters via Copper Hydride Precursors. INORGANIC CHEMISTRY 2021-06, 60, 10799-10807.
- Arif I. Inamdar, Batjargal Sainbileg, Saqib Kamal, Khasim Saheb Bayikadi, Raman Sankar, Tzuoo Tsair Luo, Michitoshi Hayashi,* Ming-Hsi Chiang,* Kuang-Lieh Lu* Water-assisted spin-flop antiferromagnetic behaviour of hydrophobic Cu-based metal–organic frameworks. DALTON TRANSACTIONS 2021-05, 50, 5754-5758.
- Jing-Xiang Xu, Kai-Ti Chu, Ming-Hsi Chiang, Jeng-Liang Han* Organocatalytic asymmetric allylic alkylation of 2-methyl-3-nitroindoles: a route to direct enantioselective functionalization of indole C(sp3)–H bonds. ORGANIC & BIOMOLECULAR CHEMISTRY 2021-02, 19, 1503-1507.
- Ming-Chuan Cheng, Gene-Hsiang Lee, Tien-Sung Lin, Yu-Chiao Liu, Ming-Hsi Chiang, Shie-Ming Peng* A new series of heteronuclear metal strings, MRhRh(dpa)4Cl2 and MRhRhM(dpa)4X2, from the reactions of Rh2(dpa)4 with metal ions of group 7 to group 12. DALTON TRANSACTIONS 2021-01, 50, 520-534.
- Ming-Hsi Chiang,* Vladimir Pelmenschikov,* Leland B. Gee, Yu-Chiao Liu, Chang-Chih Hsieh, Hongxin Wang, Yoshitaka Yoda, Hiroaki Matsuura, Lei Li, and Stephen P. Cramer* High Frequency Fe–H and Fe–H2 Modes in a trans-Fe(η2-H2)(H) Complex – A Speed Record for Nuclear Resonance Vibrational Spectroscopy. INORGANIC CHEMISTRY 2021-01, 60, 555-559.
- Tushar Sanjay Jadhav, Syed Ali Abbas, Yu-Chiao Liu, Wen-Ti Wu, Gene-Hsiang Lee, Chih-Wei Chu,* Ming-Hsi Chiang* Discrete metal-oxide clusters with organo-functionalization as high-performance anode materials. ACS Applied Energy Materials 2021-01, 4, 643-654.
- Gee, Leland Bruce; Pelmenschikov, Vladimir;* Wang, Hongxin; Mishra, Nakul; Liu, Yu-Chiao; Yoda, Yoshitaka; TAMASAKU, Kenji; Chiang, Ming-Hsi*; Cramer, Stephen P.* Vibrational characterization of a diiron bridging hydride complex – a model for hydrogen catalysis. Chemical Science 2020-07, 11, 5487-5493.
- Inamdar, Arif I.; Pathak, Abhishek; Usman, Muhammad; Chiou, Kuan-Ru; Tsai, Pei-Hsien; Mendiratta, Shruti; Kamal, Saqib; Liu, Yen-Hsiang; Chen, Jenq-Wei; Chiang, Ming-Hsi; Lu, Kuang-Lieh* Highly hydrophobic metal–organic framework for self-protecting gate dielectrics. Journal of Materials Chemistry A 2020-06, 8, 11958-11965.
- Chiang, Chuan-Kuei; Chu, Kai-Ti; Lin, Chia-Chin; Xie, Shi-Rou; Liu, Yu-Chiao; Demeshko, Serhiy; Lee, Gene-Hsiang; Meyer, Franc; Tsai, Ming-Li;* Chiang, Ming-Hsi*; Lee, Chien-Ming* Photoinduced NO and HNO Production from Mononuclear {FeNO}6 Complex Bearing a Pendant Thiol. Journal of the American Chemical Society 2020-06, 142, 8649-8661.
- Cheng, Ming-Chuan; Huang, Rui-Xiang; Liu, Yu-Chiao; Chiang, Ming-Hsi; Lee, Gene-Hsiang; Song, You; Lin, Tien-Sung; Peng, Shie-Ming* Structures and paramagnetism of five heterometallic pentanuclear metal strings containing as many as four different metals: NiPtCo2Pd(tpda)4Cl2. Dalton Transactions 2020-06, 49, 7299-7303.
- Yu-Lun Kuo, Chung-Yu Tseng, Chiao-Wei Tseng, Kai-Ti Chu, Yu-Chiao Liu, Ming-Hsi Chiang, Akinori Saeki, Yu-Tai Tao,* Hsiu-Hui Chen* Polymerization of Columnar Mesogens Tethered with Diacetylenic Side Chains. ACS Applied Polymer Materials 2020-01, 2(2), 248-255.
- Barik, S. K.; Chiu, T.-H.; Liu, Y.-C.; Chiang, M.-H.*;Gam, F.; Chantrenne, I.; Kahlal, S.; Saillard, J.-Y.*; Liu, C. W.* Mono- and hexa-palladium doped silver nanoclusters stabilized by dithiolates. NANOSCALE 2019-08, 11, 14581-14586.
- Wu, J.-Y.*; Yuan, P.-T.; Hsiao, C.-C.; Chang, H.-K.; Liu, Y.-C.; Hsu, L.-J.; Chiang, M.-H.* Structural diversity in polymeric and discrete complexes constructed by divalent transition metals and unsymmetrical quasi semirigid pyridinecarboxylate isomers. JOURNAL OF SOLID STATE CHEMISTRY 2019-07, 277, 701-712.
- Wu, W.-Y.; Hsu, C.-N.; Hsieh, C.-H.; Chiou, T.-W.*; Tsai, M.-L.*; Chiang, M.-H.; Liaw, W.-F.* NO-to-[N2O2]2–-to-N2O Conversion Triggered by {Fe(NO)2}10-{Fe(NO)2}9 Dinuclear Dinitrosyl Iron Complex. INORGANIC CHEMISTRY 2019-06, 58, 9586-9591.
- Boominathan, S. S. K.; Chang, K.-H.; Liu, Y.-C.; Wang, C.-S.; Wu, C.-F.; Chiang, M.-H.*; Chou, P.-T.*, Wu, Y.-T.* Diindeno‐fused Dibenzo[a,h]anthracene and Dibenzo[c,l]chrysene: Syntheses, Structural Analyses and Properties. CHEMISTRY-A EUROPEAN JOURNAL 2019-05, 25, 7280-7284.
- Lee, C.-M.*; Sankaralingam, M.; Chuo, C.-H.; Tseng, T.-H.; Chen, P. P.-Y.*; Chiang, M.-H.*; Li, X. X.; Lee, Y. M.; Nam, W.* A Mn(IV)–peroxo complex in the reactions with proton donors. DALTON TRANSACTIONS 2019-04, 48, 5203-5213.
- Chakrahari, K. K.; Silalahi, R. P. B.; Wang, X.; Kahlal, S.; Liu, Y.-C.; Chiang, M.-H.; Saillard, J.-Y.; Liu, C. W.* Synthesis of Bimetallic Copper-Rich Nanoclusters Encapsulating a Linear Palladium Dihydride Unit. ANGEWANDTE CHEMIE-INTERNATIONAL EDITION 2019-03, 58, 4943-4947.
- Hsieh, C.-C.; Liu, Y.-C.; Tseng, M.-C.; Chiang, M.-H.; Horng, Y.-C.* Dioxygen activation by a dinuclear thiolate-ligated Fe(II) complex. DALTON TRANSACTIONS 2019-02, 48, 379-386.
- Wong, G. F.; Yeung, L. F.; Tsoi, H. Y.; Chan, H.-S.; Chiang, M.-H.; Lee, H. K.* A Mononuclear Iron(II) Bis(guanidinate) Complex: Synthesis, Structure, and Reactivity. EUROPEAN JOURNAL OF INORGANIC CHEMISTRY 2019-01, 98-109.
- Kiran Kumarvarma Chakrahari, Rhone P. Brocha Silalahi, Jian-Hong Liao, Samia Kahlal, Yu-Chiao Liu, Jyh-Fu Lee, Ming-Hsi Chiang, Jean-Yves Saillard*, C. W. Liu* Synthesis and Structural Characterization of Inverse-Coordination Clusters from a Two-Electron Superatomic Copper Nanocluster. CHEMICAL SCIENCE 2018-09, 9, 6785-6795.
- Wan-Ting Chang, Sachil Sharma, Jian-Hong Liao, Samia Kahlal, Yu-Chiao Liu, Ming- Hsi Chiang, Jean-Yves Saillard*, Chen-Wei Liu* Heteroatom-Doping Increases Cluster Nuclearity: From An [Ag20] to An [Au3Ag18] Core. CHEMISTRY-A EUROPEAN JOURNAL 2018-09, 24, ASAP-ASAP.
- Jian-Hong Liao, Samia Kahlal, Yu-Chiao Liu, Ming-Hsi Chiang, Jean-Yves Saillard, C. W. Liu* Identification of an eight-electron superatomic cluster and its alloy in one co-crystal structure. J. Cluster Sci. 2018-09, 29, 827-835.
- Yu-Chiao Liu, Shao-An Hua*, Ming-Chuan Cheng, Li-Chung Yu, Serhiy Demeshko, Sebastian Dechert, Franc Meyer, Gene-Hsiang Lee, Ming-Hsi Chiang*, Shie-Ming Peng* Electron Delocalization of Mixed-Valence Diiron Sites Mediated by Group 10 Metal ions in Heterotrimetallic Fe−M−Fe (M = Ni, Pd and Pt) Chain Complexes. CHEMISTRY-A EUROPEAN JOURNAL 2018-08, 24, 11649-11666.
- Kai-Ti Chu, Yu-Chiao Liu*, Min-Wen Chung, Agus Riyanto Poerwoprajitno, Gene-Hsiang Lee, Ming-Hsi Chiang* Energy-Efficient Hydrogen Evolution by FeS Electrocatalysts: Mechanistic Investigations. INORGANIC CHEMISTRY 2018-07, 57, 7620-7630.
- Yan-Ru Lin, Pilli V. V. N. Kishore, Jian-Hong Liao, Samia Kahlal, Yu-Chiao Liu, Ming-Hsi Chiang, Jean-Yves Saillard*, C. W. Liu* Synthesis, Structural Characterization and Transformation of an Eight-Electron Superatomic Alloy, [Au@Ag19{S2P(OPr)2}12]. NANOSCALE 2018-04, 10, 6855-6860.
- Rhone P. Brocha Silalahi, Kiran Kumarvarma Chakrahari, Jian-Hong Liao, Samia Kahlal, Yu-Chiao Liu, Ming-Hsi Chiang, Jean-Yves Saillard*, Chen-Wei Liu* Synthesis of Two-Electron Bimetallic Cu-Ag and Cu-Au Clusters by using [Cu13(S2CNnBu2)6(C≡CPh)4]+ as a Template. CHEMISTRY-AN ASIAN JOURNAL 2018-03, 13, 500-504.
- Ming-Chuan Cheng, Shao-An Hua, Qi-ying Lv, Marc Sigrist, Gene-Hsiang Lee, Yu-Chiao Liu, Ming-Hsi Chiang, Shie-Ming Peng* Stepwise synthesis of the heterotrimetallic chains [MRu2(dpa)4X2]0/1+ using group 7 to group 12 transition metal ions and [Ru2(dpa)4Cl]. DALTON TRANSACTIONS 2018-02, 47, 1422-1434.
- Min-Wen Chung, Yu-Chiao Liu, Tao-Hung Yen, Ming-Hsi Chiang* (featured as a cover paper) Bilayer Vesicles as a Noncovalent Immobilization Platform of Electrocatalysts for Energy Conversion in Neutral Aqueous Media. CHEMELECTROCHEM 2018-01, 5, 20-24.
- Jing-Yun Wu*, Ming-Shiou Zhong, Ming-Hsi Chiang* Anion-Directed Metallocages: A Study on the Tendency of Anion Templation. CHEMISTRY-A EUROPEAN JOURNAL 2017-10, 23, 15957-15965.
- Chien-Ming Lee*, Wun-Yan Wu, Ming-Hsi Chiang*, D. Scott Bohle*, and Gene-Hsiang Lee Generation of a Mn(IV)–Peroxo or Mn(III)–Oxo–Mn(III) Species upon Oxygenation of Mono- and Binuclear Thiolate-Ligated Mn(II) Complexes. INORGANIC CHEMISTRY 2017-09, 56, 10559-10569.
- Wan-Ting Chang, Po-Yi Lee, Jian-Hong Liao, Kiran Kumarvarma Chakrahari, Samia Kahlal, Yu-Chiao Liu, Ming-Hsi Chiang, Jean-Yves Saillard*, C. W. Liu* Eight-Electron Silver and Mixed Gold/Silver Nanoclusters Stabilized by Selenium Donor Ligands. ANGEWANDTE CHEMIE-INTERNATIONAL EDITION 2017-08, 56, 10178-10182.
- Chang, Hsuan-Hao; Chu, Kai-Ti; Chiang, Ming-Hsi; Han, Jeng-Liang* Organocatalytic enantioselective Michael reaction of 1,3-dicarbonyls with α-substituted β-nitroacrylates. TETRAHEDRON 2017, 73, 727-734.
- Yen, Tao-Hung; He, Zong-Cheng; Lee, Gene-Hsiang; Tseng, Mei-Chun; Shen, Yu-Hsuan; Tseng, Tien-Wen*; Liaw, Wen-Feng*; Chiang, Ming-Hsi* Reduced thione ligation is preferred over neutral phosphine ligation in diiron biomimics regarding electronic functionality: a spectroscopic and computational investigation. CHEMICAL COMMUNICATIONS 2017, 53, 332-335.
- Wu, J.-Y.*; Cheng, F.-Y.; Chiang, M.-H.* Synthesis, Crystal Structure, and Magnetic Properties of a Two-Fold Interpenetrated Diamondoid Open Framework. JOURNAL OF SOLID STATE CHEMISTRY 2016, 242, 8-13.
- Tsou, L.-H.; Sigrist, M.; Chiang, M.-H.; Horng, E.-C.; Chen, C.-H.; Huang, S.-L.; Lee, G.-H.; Peng, S.-M.* Asymmetric Tetranuclear Nickel Chains with Unidirectionally Ordered 2-(α-(5-phenyl)pyridylamino)-1,8-naphthyridine ligands. DALTON TRANSACTIONS 2016, 45, 17281-17289.
- Dhayal, R. S.; Lin, Y.-R.; Liao, J.-H.; Chen, Y.-J.; Liu, Y.-C.; Chiang, M.-H.; Kahlal, S.; Saillard, J.-Y.*; Liu; C. W.* [Ag20{S2P(OR)2}12]: A Superatom Complex with a Chiral Metallic Core and High Potential for Isomerism. CHEMISTRY-A EUROPEAN JOURNAL 2016, 22, 9943-9947.
- Chakrahari, Kiran Kumarvarma; Liao, Jian-Hong; Kahlal, Samia; Liu, Yu-Chiao; Chiang, Ming-Hsi*; Saillard, Jean-Yves*; Liu, C. W.* [Cu13{S2CNnBu2}6(acetylide)4]+: A Two-Electron Superatom. ANGEWANDTE CHEMIE-INTERNATIONAL EDITION 2016, 55, 14704-14708.
- Liu, Y.-C.; Yen, T.-H.; Chu, K.-T.; Chiang, M.-H.* Utilization of Non-Innocent Redox Ligands in [FeFe] Hydrogenase Modeling for Hydrogen Production. COMMENTS ON INORGANIC CHEMISTRY 2016, 36, 141-181.
- Tseng, T.-W.*; Luo, T.-T.; Wu, J.-Y.; Tsai, C.-C.; Huang, C.-Y.; Chiang, M.-H.*; Lu, K.-L.* Adaptation of Guest Molecules: A Simple System that Amplifies the Gentle Perturbation of Host Lattices from Nickel(II) to Cobalt(II). INORGANICA CHIMICA ACTA 2016, 445, 96-102.
- Wu, J.-Y.*; Zhong, M.-S.; Chiang, M.-H.*; Bhattacharya, D.*; Lee, Y.-W.; Lai, L.-L. Anion-Directed Metallocages, Coordination Chain, and Complex Double Salt of Copper(II) Metallo-assemblies: Structures, Magnetic Properties, EPR and Density Functional Study. CHEMISTRY-A EUROPEAN JOURNAL 2016, 22, 7238-7247.
- Liu, Y.-C.; Chu, K.-T.; Huang, Y.-L.; Hsu, C.-H.; Lee, G.-H.; Tseng, M.-C.; Chiang, M.-H.* Protonation/Reduction of Carbonyl-Rich Diiron Complexes and the Direct Observation of Triprotonated Species: Insights into the Electrocatalytic Mechanism of Hydrogen Formation. ACS Catalysis 2016, 6, 2559-2576.
- Tseng, T.-W.*; Luo, T.-T.; Shih, Y.-R.; Shen, J.-W.; Lee, L.-W.; Chiang, M.-H.*; Lu, K.-L.* Self-triggered conformations of disulfide ensembles in coordination polymers with multiple metal clusters. CrystEngComm 2015, 17, 2847-2856.
- Dhayal, R. S.; Liao, J.-H.; Liu, Y.-C.; Chiang, M.-H.; Kahlal, S.; Saillard, J.-Y.; Liu, C. W.* [Ag21{S2P(OiPr)2}12]+: An Eight-Electron Superatom. ANGEWANDTE CHEMIE-INTERNATIONAL EDITION 2015, 54, 3702-3706.
- Chu, K.-T.; Liu, Y.-C.; Huang, Y.-L.; Lee, G.-H.; Tseng, M.-C.; Chiang, M.-H.* Redox Communication within Multinuclear Iron-Sulfur Complexes Related to Electronic Interplay in the Active Site of [FeFe]Hydrogenase. CHEMISTRY-A EUROPEAN JOURNAL 2015, 21, 6852-6861.
- Dhayal, R. S.; Liao, J.-H.; Kahlal, S.; Wang, X.; Liu, Y.-C.; Chiang, M.-H.; van Zyl, W. E.; Saillard, J.-Y.; Liu, C. W.* [Cu32(H)20{S2P(OiPr)2}12]: The Largest Number of Hydrides Recorded in a Molecular Nanocluster by Neutron Diffraction. CHEMISTRY-A EUROPEAN JOURNAL 2015, 21, 8369-8374.
- Chu, K.-T.; Liu, Y.-C.; Huang, Y.-L.; Hsu, C.-H.; Lee, G.-H.; Chiang, M.-H.* (featured as a cover paper) A Reversible Proton Relay Process Mediated by H-bonding Interaction in [FeFe]hydrogenase Modeling. CHEMISTRY-A EUROPEAN JOURNAL 2015, 21, 10978-10982.
- Dhayal, R. S.; Liao, J.-H.; Wang, X.; Liu, Y.-C.; Chiang, M.-H.; Kahlal, S.; Saillard, J.-Y.; Liu, C. W.* Diselenophosphate-Induced Conversion of an Achiral [Cu20H11{S2P(OiPr)2}9] into a Chiral [Cu20H11{Se2P(OiPr)2}9] Polyhydrido Nanocluster. ANGEWANDTE CHEMIE-INTERNATIONAL EDITION 2015, 54, 13604-13608.
- Capangpangan, R. Y.; dela Rosa, M. A. C.; Obena, R. P.; Chou, Y.-J.; Tzou, D.-L.; Shih, S.-J.; Chiang, M.-H.; Lin, C.-C.; Chen, Y.-J.* Monodispersity of Magnetic Immuno-Nanoprobes Enhances the Detection Sensitivity of Low Abundance Biomarkers in One Drop of Serum. ANALYST 2015, 140, 7678-7686.
- Capangpangan, R. Y.; dela Rosa, M. A. C.; Chang, C. H.; Wang, W. C.; Peng, J.; Shih, S. J.; Chiang, M.-H.; Tzou, D. L.; Lin, C. C.; Chen, Y. J.* Selective enrichment and sensitive detection of candidate disease biomarker using a novel surfactant-coated magnetic nanoparticles. IOP Conf. Series: Materials Science and Engineering 2014, 64, 012022-1-012022-7.
- Wu, J.-Y.; Hsiao, C.-C.; Chiang, M.-H. Concomitant Crystallization of Genuine Supramolecular Isomeric Rhombus Grid and Ribbon. CRYSTAL GROWTH & DESIGN 2014, 14, 4321-4328.
- Liu, Y.-C.; Chiang, M.-H.; Huang, Y.-L. Redox Non-innocent Ligands in Catalysis. Chemistry (The Chinese Chemical Society, Taipei) 2014, 72, 67-76.
- Edwards, A. J.; Dhayal, R. S.; Liao, P.-K.; Liao, J.-H.; Chiang, M.-H.; Piltz, R. O.; Kahlal, S.; Saillard, J.-Y.; Liu, C. W. (featured as a cover paper) Chinese Puzzle Molecule: A Fifteen Hydride, 28 Copper Nanoball. ANGEWANDTE CHEMIE-INTERNATIONAL EDITION 2014, 53, 7214-7218.
- Tsou, C.-C.; Chiu, W.-C.; Ke, C.-H.; Tsai, J.-C.; Wang, Y.-M.; Chiang, M.-H.; Liaw, W.-F. Iron(III) Bound by Hydrosulfide Anion Ligands: NO-Promoted Stabilization of the [FeIII–SH] Motif. JOURNAL OF THE AMERICAN CHEMICAL SOCIETY 2014, 136, 9424-9433.
- Liu, Y.-C.; Chiang, M.-H.* Photocatalytic Hydrogen Production Based on [FeFe]-Hydrogenase Mimics. Chemistry (The Chinese Chemical Society, Taipei) 2013, 71, 299-309.
- Yen, T.-H.; Chu, K.-T.; Chiu, W.-W.; Chien, Y.-C.; Lee, G.-H.; Chiang, M.-H. (Invited contribution to a special issue) Synthesis and characterization of the diiron biomimics bearing phosphine borane for hydrogen formation. POLYHEDRON 2013, 64, 247-254.
- Liu, Y.-C.; Chu, K.-T.; Jhang, R.-L.; Lee, G.-H.; Chiang, M.-H.* [FeFe] Hydrogenase Active Site Modeling: a Key Intermediate Bearing a Thiolate Proton and Fe Hydride. CHEMICAL COMMUNICATIONS 2013, 49, 4743-4749.
- Fu-Te Tsai,* Yu-Ching Lee, Ming-Hsi Chiang, Wen-Feng Liaw* Nitrate-to-Nitrite-to-Nitric Oxide Conversion Modulated by Nitrate-Containing {Fe(NO)2}9 Dinitrosyl Iron Complex (DNIC). INORGANIC CHEMISTRY 2013, 52, 464-473.
- Chien-Ming Lee*, Chi-He Chuo, Ching-Hui Chen, Cho-Chun Hu, Ming-Hsi Chiang*, Yu-Jan Tseng, Ching-Han Hu, Gene-Hsiang Lee Structural and Spectroscopic Characterization of a Monomeric Side-On Manganese(IV) Peroxo Complex. ANGEWANDTE CHEMIE-INTERNATIONAL EDITION 2012, 51, 5427-5430.
- Wei-Chih Shih, Tsai-Te Lu, Li-Bo Yang, Fu-Te Tsai, Ming-Hsi Chiang*, Jyh-Fu Lee, Yun-Wei Chiang, Wen-Feng Liaw* New Members of a Class of Dinitrosyliron Complexes (DNICs): The Characteristic EPR Signal of the Six-Coordinate and Five-Coordinate {Fe(NO)2}9 DNICs. JOURNAL OF INORGANIC BIOCHEMISTRY 2012, 113, 83-93.
- Yu-Chiao Liu, Tao-Hung Yen, Yu-Jan Tseng, Ching-Han Hu, Gene-Hsiang Lee, Ming-Hsi Chiang* Electron Delocalization from the Fullerene Attachment to the Diiron Core within the Active-Site Mimics of [FeFe]Hydrogenase. INORGANIC CHEMISTRY 2012, 51, 5997-5999.
- Meng-Jung Tsai, Jing-Yun Wu*, Ming-Hsi Chiang*, Cheng-Hao Huang, Ming-Yu Kuo, Long-Li Lai* Infinite Copper(II) Coordination Architectures from a Resonative Aminotriazine-Derived Tripodal Ligand: Synthesis, Structures, and Magnetic Properties. INORGANIC CHEMISTRY 2012, 51, 12360-12371.
- Jing-Yun Wu*, Ming-Shiou Zhong, Ming-Hsi Chiang, Meng-Rong Tsai, Long-Li Lai Synthesis, Characterization and Solvent-Mediated Structural Transformation of a Discrete Tetragonal Metalloprism. DALTON TRANSACTIONS 2012, 41, 156-164.
- Shao-Hsuan Lin, Chen-I Yang, Ting-Shen Kuo, Ming-Hsi Chiang, Kung-Chung Hsu, Kuang-Lieh Lu* Self-Adaptation of Manganese Chloride Arrangement toward High Spin Mn5(u-Cl)4 Cluster-Based Metal Organic Framework with S = 15/2. DALTON TRANSACTIONS 2012, 41, 1448-1450.
- Yu-Chiao Liu, Chia-Hsin Lee, Gene-Hsiang Lee, Ming-Hsi Chiang* (Invited contribution to a special issue) Influence of the Attached Redox-Active Phosphine on Oxidations of the Diiron Core Related to the Active Site of Fe-Only Hydrogenase. EUROPEAN JOURNAL OF INORGANIC CHEMISTRY 2011, 2011, 1151-1162.
- Yu-Chiao Liu, Tao-Hung Yen, Ming-Hsi Chiang* Hydrogen Activation and Biological Inorganic Mimics of Hydrogenases. Chemistry (the Chinese Chemical Society, Taipei) 2011, 第69卷,頁87-105.
- Yu-Chiao Liu, Ling-Kuang Tu, Tao-Hung Yen, Gene-Hsiang Lee, Ming-Hsi Chiang* Influences on the Rotated Structure of the Diiron Dithiolate Complexes: Electronic Asymmetry vs. Secondary Coordination Sphere Interaction. DALTON TRANSACTIONS 2011, 40, 2528-2541.
- Pei-Chin Lin, Hsing-Yin Chen, Po-Yu Chen, Ming-Hsi Chiang, Michael Y. Chiang, Ting-Shen Kuo, Sodio C. N. Hsu* Self-Assembly and Redox Modulation of the Cavity Size of an Unusual Rectangular Iron Thiolate Aryldiisocyanide Metallocyclophane. INORGANIC CHEMISTRY 2011, 50, 10825-10834.
- Jing-Yun Wu*, Sheng-Ming Huang, Ming-Hsi Chiang Hydro(solvo)thermal Synthesis of Homochiral Metal–Camphorate Coordination. CrystEngComm 2010, 12, 3909-3913.
- Yu-Chiao Liu, Ling-Kuang Tu, Tao-Hung Yen, Gene-Hsiang Lee, Shu-Ting Yang, Ming-Hsi Chiang* Secondary Coordination Sphere Interactions within the Biomimetic Iron Azadithiolate Complexes Related to Fe-Only Hydrogenase: Dynamic Measure of Electron Density about the Fe Sites. INORGANIC CHEMISTRY 2010, 49, 6409-6420.
- Ming-Hsi Chiang*, Yu-Chiao Liu, Shu-Ting Yang, Gene-Hsiang Lee Biomimetic Model Featuring the NH Proton and Bridging Hydride Related to a Proposed Intermediate in Enzymatic H2 Production by Fe-Only Hydrogenase. INORGANIC CHEMISTRY 2009, 48, 7604-7612.
- Mark R. Antonio, Ming-Hsi Chiang, Soenke Seifert, David M. Tiede, Pappannan Thiyagarajan In Situ Measurement of the Preyssler Polyoxometalate Morphology upon Electrochemical Reduction: a Redox System with Born Electrostatic Ion Solvation Behavior. JOURNAL OF ELECTROANALYTICAL CHEMISTRY 2009, 626, 103-110.
- Mark R. Antonio, Ming-Hsi Chiang Stabilization of Plutonium(III) in the Preyssler Polyoxometalate. INORGANIC CHEMISTRY 2008, 47, 8278-8285.
- Tai-Nan Chen, Feng-Chun Lo, Ming-Li Tsai, Ko-Nien Shih, Ming-Hsi Chiang, Gene-Hsiang Lee, Wen-Feng Liaw* Dinitrosyl Iron Complexes [E5Fe(NO)2]- (E = S, Se): A Precursor of Roussin's Black Salt [Fe4E3(NO)7]. INORGANICA CHIMICA ACTA 2006, 359, 2525-2533.
- Fu-Te Tsai, Show-Jen Chiou, Ming-Che Tsai, Ming-Li Tsai, Hsiao-Wen Huang, Ming-Hsi Chiang, Wen-Feng Liaw* Dinitrosyl Iron Complexes (DNICs) [L2Fe(NO)2]- (L = Thiolate): Interconversion among {Fe(NO)2}9 DNICs, {Fe(NO)2}10 DNICs, and [2Fe-2S] Clusters, and the Critical Role of the Thiolate Ligands in Regulating NO Release of DNICs. INORGANIC CHEMISTRY 2005, 44, 5872-5881.
- Hao-Wen Chen, Chin-Wei Lin, Chiao-Chun Chen, Li-Bo Yang, Ming-Hsi Chiang, Wen-Feng Liaw* Homodinuclear Iron Thiolate Nitrosyl Compounds [(ON)Fe(S,S-C6H4)2Fe(NO)2]- and [(ON)Fe(SO2,S-C6H4)(S,S-C6H4)Fe(NO)2]- with {Fe(NO)}7-{Fe(NO)2}9 Electronic Coupling: New Members of a Class of Dinitrosyl Iron Complexes. INORGANIC CHEMISTRY 2005, 44, 3226-3232.
- Drew Gorman-Lewis, Jeremy B. Fein*, Lynne Soderholm, Mark P. Jensen, Ming-Hsi Chiang Experimental Study of Neptunyl Adsorption onto Bacillus Subtilis. GEOCHIMICA ET COSMOCHIMICA ACTA 2005, 69, 4837-4844.
- Ming-Hsi Chiang, Mark R. Antonio*, Lynne Soderholm Energetics of the Preyssler Anion’s Molecular Orbitals: Quantifying the Effect of the Encapsulated-Cation’s Charge. DALTON TRANSACTIONS 2004, 2004, 3562-3567.
- Ming-Hsi Chiang, Mark R. Antonio*, Clayton W. Williams, Lynne Soderholm A Unique Coordination Environment for an Ion: EXAFS Studies and Bond Valence Model Approach of the Encapsulated Cation in the Preyssler Anion. DALTON TRANSACTIONS 2004, 2004, 801-806.
- Ming-Hsi Chiang, Juile A. Dzielawa, Mark L. Dietz, Mark R. Antonio* Redox Chemistry of the Keggin Heteropolyoxotungstate Anion in Ionic Liquids. JOURNAL OF ELECTROANALYTICAL CHEMISTRY 2004, 567, 77-84.
- Mark R. Antonio*, Ming-Hsi Chiang, Clayton W. Williams, Lynne Soderholm In Situ Actinide X-ray Absorption Spectroelectrochemistry. MRS Proc. 2004, 802, 157-168.
- Skantha Skanthakumar, Drew Gorman-Lewis, Andrew J. Locock, Ming-Hsi Chiang, Mark P. Jensen, Peter C. Burns, Jeremey Fein, Charles D. Jonah, Klaus Attenkofer, Lynne Soderholm Changing Np Redox Speciation in the Synchrotron Beam. MRS Proc. 2003, 802, 151-156.
- Ming-Hsi Chiang, Lynne Soderholm, Mark R. Antonio Redox Chemistry of Actinide Ions in Wells-Dawson Heteropolyoxoanion Complexes. EUROPEAN JOURNAL OF INORGANIC CHEMISTRY 2003, 2003, 2929-2936.
- Ming-Hsi Chiang, Clayton W. Williams, Lynne Soderholm, Mark R. Antonio* Coordination of Actinide Ions in Wells-Dawson Heteropolyoxoanion Complexes. EUROPEAN JOURNAL OF INORGANIC CHEMISTRY 2003, 2003, 2663-2669.
Chapter of Publication
- Yu-Chiao Liu, Tao-Hung Yen, Ling-Kuang Tu, Ming-Hsi Chiang*, 2011, “Design of Biomimetic Models Related to the Active Sites of Fe-Only Hydrogenase”, editor(s): Anne George, Biomimetic Based Applications, pp. 123-140, Austria: InTech Publishing.
Paper of Conference (or Symposium)
- Ming-Hsi Chiang, 2016, “A Concerted Proton-Electron Transfer-Involved Catalysis by Fe-S Electrocatalysts for Hydrogen Evolution”, paper presented at 8th Asian Biological Inorganic Chemistry Conference, Auckland, New Zealand: IBICS, 2016-12-04 ~ 2016-12-09.
- Ming-Hsi Chiang, 2016, “Diiron Electrocatalyst for Hydrogen Production”, paper presented at 3rd International Conference on Organometallics and Catalysis, Seoul, Korea: Seoul National University, 2016-08-28 ~ 2016-08-30.
- Yu-Chiao Liu, Kai-Ti Chu, Ming-Hsi Chiang*, 2015, “A High-Performance Diiron Electrocatalyst for Hydrogen Production”, paper presented at 17th International Conference on Biological Inorganic Chemistry, Beijing, China: IBICS, 2015-07-20 ~ 2015-07-24.
- Ming-Hsi Chiang, 2015, “A High-Performance Diiron Electrocatalyst for Hydrogen Production”, paper presented at 6th North America-Greece-Cyprus Workshop on Paramagnetic Materials, Athens, Greece: University of Florida, 2015-06-03 ~ 2015-06-05.
- Yu-Chiao Liu, Kai-Ti Chu, Gene-Hsiang Lee, and Ming-Hsi Chiang, 2014, “A High-Performance Diiron Electrocatalyst for Hydrogen Production”, paper presented at The 8th Taiwan-Japan Bilateral Symposium on Architecture of Functional Organic Molecules, Fukuoka, Japan: Kyushu University, 2014-11-27 ~ 2014-11-29.
- Kai-Ti Chu, Yu-Chiao Liu, Ruei-Lin Jhang, Gene-Hsiang Lee, Ming-Hsi Chiang, 2013, “[FeFe] hydrogenase Active Site Modeling: a Key Intermediate Bearing a Thiolate Proton and Fe Hydride”, paper presented at The ISACS12: Challenges in Chemical Renewable Energy, Cambridge, UK: RSC, 2013-09-03 ~ 2013-09-06.
- Ming-Hsi Chiang, 2013, “Tuning Electronic Structure of the Fe Core Within FeS Biomimics for Efficient Hydrogen Production”, paper presented at The International Symposium on Bioinorganic Chemistry, Korea, Seoul: Society of Korean Bioinorganic Chemistry, 2013-06-12 ~ 2013-06-15.
- Ming-Hsi Chiang, 2013, “Modulation of the Electronic Structure of the Fe2 Core Related to [FeFe] Hydrogenases for Efficient Hydrogen Production”, paper presented at The 5th NAGC WORKSHOP on Paramagnetic Materials, Limassol, Cyprus: University of Florida, 2013-05-22 ~ 2013-05-26.
- Yu-Chiao Liu, Tao-Hung Yen, Ming-Hsi Chiang, 2012, “Electron Delocalization from the Fullerene Attachment to the Diiron Core”, paper presented at The 6th Asian Biological Inorganic Chemistry Conference, Hong Kong: SBIC, 2012-11-05 ~ 2012-11-08.
- Ming-Hsi Chiang, 2012, “Electron Delocalization from the Fullerene Attachment to the Diiron Core”, paper presented at The Tateshina Conference, Nagano, Japan: The University of Tokyo, 2012-11-09 ~ 2012-11-11.
- Kai-Ti Chu, Yu-Chiao Liu, Chia-Hsin Lee, Gene-Hsiang Lee, Ming-Hsi Chiang, 2012, “Influence of a Redox-Active Phosphane Ligand on the Oxidations of a Diiron Core Related to the Active Site of [FeFe] Hydrogenase”, paper presented at The 3rd International Symposium on Solar Cells and Solar Fuels, Dalian, China: Dalian University of Technology, 2012-09-07 ~ 2012-09-11.
- Tao-Hung Yen, Yu-Chiao Liu, Ling-Kuang Tu, Gene-Hsiang Lee, Ming-Hsi Chiang, 2011, “The Influences of Secondary Coordination Sphere Interaction on Electronic Asymmetry within the Biomimetic Iron Azadithiolate Complexes related to the Active Site of Fe-only Hydrogenase”, paper presented at The 15th International Conference of Biological Inorganic Chemistry, Vancouver, Canada: SBIC, 2011-08-07 ~ 2011-08-12.
- Yu-Chiao Liu, Ling-Kuang Tu, Tao-Hung Yen, Gene-Hsiang Lee and Ming-Hsi Chiang*, 2010, “Dynamic measure of electron density about the Fe sites within the biomimetic iron azadithiolate complexes related to Fe-only hydrogenase”, paper presented at The 5th Asian Biological Inorganic Conference, Kaohsiung, Taiwan: Taiwan biological inorganic society, 2010-11-01 ~ 2010-11-05.
- Yu-Chiao Liu, Ling-Kuang Tu, Tao-Hung Yen, Gene-Hsiang Lee and Ming-Hsi Chiang*, 2010, “Dynamic measure of electron density about the Fe sites within the biomimetic iron azadithiolate complexes related to Fe-only hydrogenase”, paper presented at 24th ICOMC 2010, Taipei, Taiwan: NTU, NTHU, TKU, NSC, 2010-07-18 ~ 2010-07-23.
Patent
- 鐵硫錯合物及其作為觸媒用以產氫之方法IRON-SULFUR COMPLEX AND METHOD FOR PRODUCING HYDROGEN USING THE SAME AS CATALYST
Taiwan:I640530 2018 - 高效產之鐵硫分子觸媒 IRON-SULFUR COMPLEX AND METHOD FOR PRODUCING HYDROGEN USING THE SAME AS CATALYST
USA-regular:US10,016,748B2 2018


