中央研究院化學研究所-人員

目前我們實驗室發展的研究主題是利用過渡金屬錯合物或有機金屬為材料,尋找這些分子於催化反應中新的應用,以發展出新的催化化學,並將有利於綠色化學的發展。
(a) Para-CH bond Activation of Pyridine with Isolation Ni-Al Intermediate
- Journal of the American Chemical Society, 2010, 132, 11887.
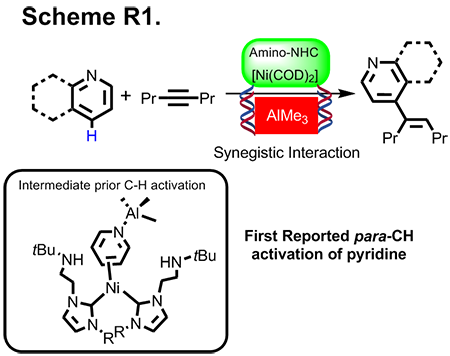
We discovered that the first example of bimetallic Ni-Al catalyst mediated the para-C-H functionalization of pyridinein a selective manner (Scheme R1). In similar studies, we also delivered penetrating new insight on the synergistic effect of a bimetallic in C-H bond activation based on single crystal X-ray diffraction experiment. The success of this program hinges upon a new conceptual paradigm: (a) Acceleration on one of the key steps in catalytic CH activation via Lewis acid, and (b) Steric demanding created by Lewis metal for reinforcing positional selectivity. Our work has been published in Journal of the American Chemical Society. Central to the implication on our discovery is pervasive (total citations = 61 times), as many researchers have implemented our bimetallic concept into their catalysis effort.
(b) Double CH Activation
- Chemical Communication, 2014, 50, 3671-3673
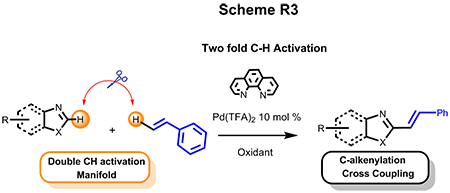
In the interest of constructing C-C bonds in a more atom- and step-economical fashion, we also developed efficient Pd-catalyzed double C-H activation of cross coupling of heteroarenes with styrenes and other olefinic substrates. This alkenylation paradigm encompasses a wide range of substrate scopes and provides a straightforward and high valued synthetic approach toward C2-E-alkenylated azole motifs. This work has been selected as the highlight in the back cover of the Chemical Communication.
(c) Functional Linked Amino-NHC: Organic Catalysis and Medicinal Implications
- J. Med. Chem. 2011, 54, 5245-5249.
- Chemical Communication, 2012, 48, 6702-6704.
- Chem. Asian J. 2011, 6, 1520-1524.
- Organometallics, 2009, 28, 1060-1067
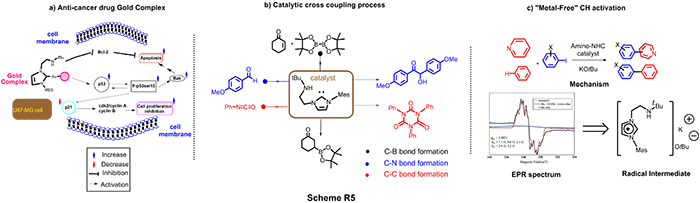
Our lab is a pioneer of designing a myriad of amino-NHC frameworks and investigating of their fundamental chemical reactivities, which we have made numerous significant contributions and innovations in the context of molecular catalysis, synthetic chemistry, medicinal and main group elements as illustrated in Scheme R5 with high number of citation (150 times). For example, Anti-cancer agents: Amino-NHC Gold Complexes (Scheme R5a) was developed to have the capacity to induce apoptosis through a p53-bak pathway, a sensational finding that could serve as a new alternate strategy to reduce the resistance of cancer cells to p53-induced apoptosis. The anti-tumor pathway is uniquely different from classical drug cis-platin.
We also have used Amino-NHC to perform “Metal-Free” catalytic process. For the first time, amino-NHC has been shown to mediate the direct C–H functionalization of benzene and pyridine in the absence of a metal catalyst (Scheme R5(c)). Using Electron Spin Resonance, a state of art technique, we have demonstrated the first spectroscopic evidence confirming the radical intermediate in this process, setting a ground breaking work for many other works in this field, namely “metal-free” catalytic C-H bond activation. Furthermore, this versatility of amino-NHC catalyst is also witnessed in C-C, C-B and C-N bond formation (Scheme R5(b)).
(d) Creative Ligand Design Approach: Carbodicarbene and Elusive Boron species
- Journal of the American Chemical Society, 2014, 136, 914-917.
- Organometallics, 2013, 32, 2435-2442
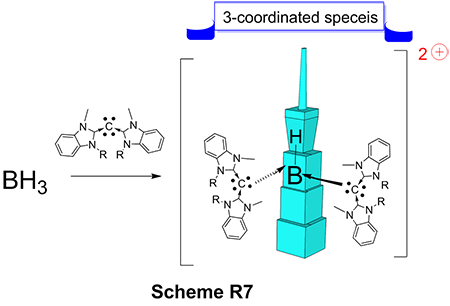
Recently, we have designed another novel ligand with interesting framework called carbodicarbene. In our main group metal’s endeavor, we have discovered the formation of a hitherto unknown three-coordinate dicationic hydrido boron complex with unique bonding environment (Scheme 5). Supporting ligand carbodicarbene gave unprecedented reaction with BH3 without using more highly electrophilic Lewis acid precursors. A reaction behavior not observed for other common NHC ligands. These results pave the way for future studies in highly electrophilic Lewis acid chemistry with interesting potential applications in organic synthesis and catalysis.
(e) Isolation of Novel Pincer bis-(pyridine)carbodicarbene Framework and Highly Efficient Cross-Coupling
- Angew. Chem. Int. Ed. 2014, Just Accepted
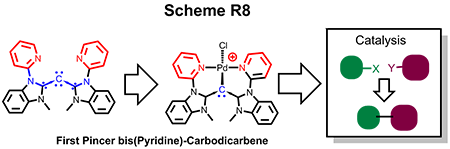
The simple synthetic development of acyclic pincer bis(pyridine)carbodicarbene is realized on our endeavor on the ligand design. Presented is the first isolated structural pincer carbodicarbene with a C-C-C angle of 143°, larger than the monodentate framework. More importantly theoretical analysis revealed that this carbodicarbene embodies a more allene-like character. Pd complexes supported by this pincer ligand are active catalysts for Heck-Mizoroki and Suzuki-Miyaura coupling reactions
(f) Expanding Ligand Framework Diversity of Carbodicarbenes and Direct Observation of Boron Activation in Methylation of Amines with CO2
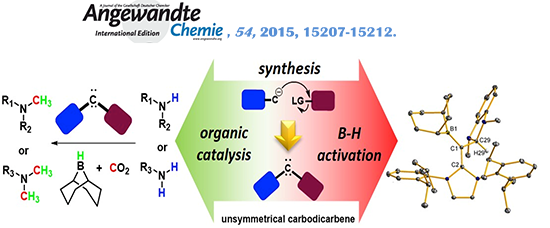
A simple and convergent synthetic strategy was developed to increase the diversity of the carbodicarbene framework by incorporation of unsymmetrical units. Reactivity studies revealed that carbodicarbenes are competent organocatalysts for amine methylation using CO2 as a synthon.
(g) Isolation of Tridentate Acyclic Bis(Pyridine)carbodicarbene and Further Studies on Its Structural Implications and Reactivities
- Angew. Chem. Int. Ed. 2015, 54, 2420.
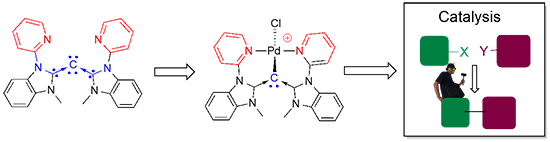
The simple synthetic development of acyclic pincer bis(pyridine)carbodicarbene is depicted herein. Presented is the first isolated structural pincer carbodicarbene with a C-C-C angle of 143°, larger than the monodentate framework. More importantly theoretical analysis revealed that this carbodicarbene embodies a more allene-like character. Pd complexes supported by this pincer ligand are active catalysts for Heck-Mizoroki and Suzuki-Miyaura coupling reactions.
- "科技部硏究傑出奬" (2019)
- " 第十五屆 水木化學文教基金會傑出青年學者獎" (2014-04)
- "Asian International Symposium Lecture Award by Chemical Society of Japan" (2013-03)
- "中國化學會傑出青年化學獎章" (2010-12)
- Hsu, Y.-C.; Shen, J.-S.; Lin, B.-C.; Chen, W.-C.; Chan, Y.-T.; Ching, W.-M.; Yap, G. P. A.; Hsu, C.-P.; Ong, T.-G. Isolation of Tridentate Acyclic Bis(Pyridine)carbodicarbene and Further Studies on Its Structural Implications and Reactivities. Angew. Chem. Int. Ed. 2014, Accepted for publication.
- Yu, M.-S.; Lee, W.-C.; Chen, C.-H.; Tsai, F.-Y.; Ong, T.-G. Controlled Regiodivergent C-H Bond Activation of Imidazo[1,5-a]pyridine via Synergistic Cooperation between Aluminum and Nickel. Organic Letters, 2014, Accepted for publication. .
- Chen, W.-C.; Lai, Y.-C.; Shih, W.-C.; Yu, M.-S.; Yap, G. P. A.; Ong, T.-G. Mechanistic Study of the Regioselective Switch for Hydroheteroarylation of Styrene Catalyzed by Bimetallic Ni-Al via C-H Activation. Chem.-Eur. J. 2014, 20, 8099.
- Huang, H.-J.; Lee, W.-C.; Yap, G. P. A.; Ong, T.-G. Synthesis and Characterization of Amino-NHC Coinage Metal Complexes and Application for C-H Activation of Caffeine. J. Organomet. Chem. 2014, 761, 64.
- Tai, C.-C; Yu, M.-S.; Chen, Y.-L.; Chuang, W.-H.; Lin T.-H.; Yap, G. P. A.; Ong, T.-G. Synthesis of a Guanidine NHC complex and its Application in Borylation Reactions. Chem. Commun. 2014, 50, 4344.
- Lee, W.-C.; Wang, T.-H.; Ong, T.-G. Ligand promoted Pd-catalyzed dehydrogenative alkenylation of hetereoarenes. Chem. Commun. 2014, 50, 3671.
- Chen, C.-C.; Lee, C.-Y.; Lin, B.-C.; Hsu, Y.-C.; Shen, J.-S.; Hsu, C.-P.; Yap, G. P. A.; Ong, T.-G. The Elusive Three-Coordinate Dicationic Hydrido Boron Complex. J. Am. Chem. Soc. 2014, 136, 914.
- Lee, W.-C.; Wang, C.-H.; Lin Y.-H.; Shih, W.-C.; Ong, T.-G. Tandem Isomerization and C–H Activation: Regioselective Hydroheteroarylation of Allylarenes. Organic Letters, 2013, 15, 5358.
- Chen, C. T.; Chen, C. H.; Ong, T.-G. Complementary Helicity Interchange of Optically Switchable Supramolecular-Enantiomeric Helicenes with (−)-Gel-Sol-(+)-Gel Transition Ternary Logic. J. Am. Chem. Soc 2013, 135, 5294.
- Chen, W. C.; Hsu, Y. C.; Lee, C. Y.; Yap, G. P. A.; Ong, T.-G. Synthetic Modification of Acyclic Bent Allenes (Carbodicarbenes) and Further Studies on Their Structural Implications and Reactivities. Organometallics, 2013, 32, 2435.
- Titel, J.; Chen, W. C.; Michel, S.; Korobkov, I.; Ong, T.-G. Richeson, D. S. Solid-State Thermolysis of a fac-Rhenium(I) Carbonyl Complex with a Redox Non-Innocent Pincer Ligand. Chem.-Eur. J. 2013, 19, 4278.
- Chen, W. C.; Hsu, Y. C.; Shih, W. C.; Lee, C. Y.; Chuang, W. H.; Tsai, Y. F.; Chen, P. P. Y.; Ong, T.-G. Metal-free arylation of benzene and pyridine promoted by amino-linked nitrogen heterocyclic carbenes. Chem. Commun. 2012, 48, 6702.
- Liu, Y. M.; Lin, Y. C.; Chen, W. C.; Cheng, J. H.; Yap, G. P. A. Sun S. S; Ong, T.-G. Synthesis and Characterization of a para-Pyridine Linked NHC Palladium Complexes and their Studies for Heck-Mizoroki Coupling Reaction. Dalton Transactions, 2012, 41, 7382.
- Shih, W. C.; Chen, W. C.; Lai, Y. C.; Yu, M. Y.; Ho, J. J.; Yap, G. P. A.; Ong, T.-G. The Regioselective Switch for Amino-NHC Mediated C-H Activation of Benzimidazole via Ni-Al Synergistically Catalysis. Organic Letters, 2012, 14, 2046.
- Tai, C. –C.; Chang, Y. –T.; Tsai, J. –H.; Jurca, T.; Yap, G. P. A.; Ong, T. –G. Subtle Reactivities of Boron and Aluminum Complexes with Amino-Linked N-Heterocyclic Carbene Ligation. Organometallics, 2012, 31, 637.
- Wang, C.-H; Shih, W.-C.; Chang, H.-C.; Kuo, E.; Hung, W.-C.; Ong, T.-G.; Li, W.-S. Preparation and Characterization of Amino-Linked Heterocyclic Carbene Palladium, Gold, and Silver Complexes: Applications in Anticancer Agents via Triggering Apoptotic Cell Death. J. Med. Chem. 2011, 54, 5245.
- Li, C.-Y.; Kuo, Y.-Y; Tsai, J.-H.; Yap, G. P. A.; Ong, T.-G. Amine-Linked N-Heterocyclic Carbene: The importance of an Pendant Free Amine Auxiliary in Assisting the Catalytic Reaction. Chem. Asian J. 2011, 6, 1520.
- Tsai, J.-H.; Lin, S.-T.; Yang, R. B.G.; Yap, G. P. A.; Ong, T.-G. The Two-way Street Transformation of Boronium and Borane Complexes Facilitated by Amino-Linked N-Heterocyclic Carbene. Organometallics 2010, 29, 4004.
- Tsai, C. –C.; Shih, W.-C.; Fang, C.-H.; Li, C.-Y.; Ong, T.-G.; Yap, G. P. A. Bimetallic Nickel Aluminun Mediated Para-Selective Alkenylation of Pyridine: Direct Observation of η2,η1-Pyridine Ni(0)−Al(III) Intermediates Prior to C−H Bond Activation. J. Am. Chem. Soc. 2010, 132, 11887.
- Hu, Y.-C.; Liang, C.-F.; Tsai, J.-H.; Yap, G. P. A.; Chang, Y.-T.; Ong, T.-G. Zirconium Complexes Supported by Imidazolones: Synthesis, Characterization, and Application of Precatalysts for the Hydroamination of Aminoalkenes. Organometallics 2010, 29, 3357.
- Hu, Y.-C.; Tsai, C.-C.; Shih, W.-C.; Yap, G. P. A.; Ong, T.-G. The Zirconium Benzyl Mediated C-N Bond Cleavage of an Amino-Linked N-Heterocyclic Carbene. Organometallics 2010, 29, 516.
- Shih, W. -C.; Wang, C.-H.; Chang, T.-T.; Yap, G. P. A.; Ong, T-G. Synthesis and Structure of an Amino- Linked N-Heterocyclic Carbene and the Reactivity of its Aluminum Adduct. Organometallics 2009, 47, 1060.
- Huang, Y.-P.; Tsai, C.-C.; Shih, W.-C.; Chang, Y.-C.; Lin, S.-T.; Yap, G. P. A.; Chao, I.; Ong, T-G. Kinetic and Thermodynamic Study of Syn—Anti Isomerization of Nickel Complexes Bearing Amino-Linked N-Heterocyclic Carbene Ligands: The Effect of the Pendant Arm of the NHC. Organometallics 2009, 28, 4316.
- Rowley, C. N.; Ong, T-G.; Priem, J.; Richeson, D. S.; Woo, T. K. Analysis of the Critical Step in Catalytic Carbodiimide Transformation: Proton Transfer from Amines, Phosphines, and Alkynes to Guanidinates, Phosphaguanidinates, and Propiolamidinates with Li and Al Catalysts. Inorg. Chem. 2008, 47, 12024.
- Rowley, C. N.; Ong, T-G.; Priem, J.; Woo, T. K.; Richeson, D. S. Amidolithium and Amidoaluminum Catalyzed Synthesis of Substituted Guanidines: An Interplay of DFT Modeling and Experiment. Inorg. Chem. 2008, 47, 9660.
- Lavoie, N.; Ong, T. G.; Gorelsky, S. I.; Korobkov, I.; Yap, G. P. A.; Richeson, D. S. Bis(imido) W(VI) complexes chelated by N,N '-Disubstituted 1,8-diamidonaphthalene: An analysis of bonding, isocyanate insertion, and Al-Me transfer. Organometallics 2007, 26, 6586.
- Ong, T. G.; O'Brien, J. S.; Korobkov, I.; Richeson, D. S. Facile and atom-efficient amidolithium-catalyzed C-C and C-N formation for the construction of substituted guanidines and propiolamidines. Organometallics 2006, 25, 4728.
- Said, F. F.; Ong, T. G.; Bazinet, P.; Yap, G. P. A.; Richeson, D. S. Linking hydrogen dicarboxylate synthons with substituted guanidinium cations: Transforming rings and chains into two- and three-dimensional structures. Cryst. Growth Des. 2006, 6, 1848.
- Said, F. F.; Bazinet, P.; Ong, T.-G.; Yap, G. P. A.; Richeson, D. S. Hydrogen Bonding Motifs of N,N',N' '-Trisubstituted Guanidinium Cations with Spherical and Rodlike Monoanions: Syntheses and Structures of I-, I3-, and SCN- Salts. Cryst. Growth Des. 2006, 6, 258.
- Said, F. F.; Ong, T.-G.; Yap, G. P. A.; Richeson, D. Strong and Weak Hydrogen-Bonding Interactions in the Structures of N,N',N' '-Trisubstituted Guanidinium Chlorides and Bromides. Cryst. Growth Des. 2005, 5, 1881.
- Ong, T. G.; Yap, G.; Richeson, D. S. Catalytic C-N bond metathesis of carbodiimides by group 4 and 5 imido complexes supported by guanidinate ligands. Chem. Commun. 2003, 2612.
- Ong, T. G.; Yap, G.; Richeson, D. S. Catalytic Construction and Deconstruction of Guanidine: Ti-Mediated Guanylation of Amines and Transamination of Guanidines. J. Am. Chem. Soc. 2003, 125, 8100.
- Ong, T. G.; Yap, G.; Richeson, D. S. Redefining the Coordination Geometry and Reactivity Guanidinate Complexes by Covalently Linking the Guanidine ligands. Organometallics 2003, 22, 387.
- Ong, T. G.; Yap, G.; Richeson, D. S. Formation of a Guanidinate-Supported Titanium Imido Complex: A Catalyst for Alkyne Hydroamination. Organometallics 2002, 21, 2839.
- Ong, T. G.; Wood, D.; Yap, G.; Richeson, D. S. Transformations of Aryl Isocyanide on Guanidinate-Supported Organozirconium Complexes To Yield Terminal Imido, Iminoacyl, and Enediamido Ligands. Organometallics 2002, 21, 1.
- Ong, T.-G.; Yap, G. P. A.; Richeson, D. S. Formation of a Guanidinate-Supported Titanium Imido Complex: A Catalyst for Alkyne Hydroamination. Organometallics 2002, 21, 2839.
Update: 2016-01-06
- Ong Tiow-Gan*, Yu Cheng-Han, Hsiao Yu-Wen, Löffler Julian, Kaiser Nicolas, Huang Bo-Hong, Lee Chao-Hsien, Hung Chen-Hsun, Shen Jiun-Shian, Yap Glenn P. A., Gessner Viktoria H.* Increasing the Donor Strength of Alkenylphosphines by Twisting the C=C Double Bond. Angewandte Chemie International Edition 2024-11-11, 63, e202416764.
- Lorianne R.Shultz-Johnson, Azina Rahmani, Johannes Frisch, Tzung-En Hsieh, Lin Hu, Jaynlynn Sosa, Marie Davy, Shaohua Xie, Melanie J. Beazley, Zhengning Gao, Pooria Golvari, Ting-Hsuan Wang, Tiow-Gan Ong, Nicholas G. Rudawski, Fudong Liu, Parag Banerjee, Xiaofeng Feng, Marcus Bär*, Titel Jurca* Modifying the Substrate-Dependent Pd/Fe<sub>2</sub>O<sub>3</sub> Catalyst–Support Synergism with ZnO Atomic Layer Deposition. ACS Applied Materials & Interfaces 2024-07-20, 16(30), 39387-39398.
- Huang Rou‐Jie, Ong Tiow‐Gan, Chein Rong‐Jie Total synthesis of cassane-type diterpenoid pikrosalvin. Journal of the Chinese Chemical Society 2023-11, .
- Yu Cheng‐Han, Au‐Yeung Ka‐Chun, Liu Ruiqin, Lee Chao‐Hsien, Jiang Dandan, Semagne Aweke Bamlaku, Wu Chia‐Hung, Wang Yu‐Jou, Wang Ting‐Hsuan, Voon Kong Kien, Yap Glenn P. A., Chen Wen‐Ching*, Frenking Gernot*, Zhao Lili*, Ong Tiow‐Gan* Diversification of the Carbodicarbene Class by Embedding an Anionic Component in its Scaffold. Chemistry – A European Journal 2023-11, 29, e202302886.
- Aweke Bamlaku Semagne, Yu Cheng-Han, Shen Jiun-Shian, Wang Sheng, Yap Glenn P. A., Chen Wen-Ching*, Ong Tiow-Gan* Binuclear Macrocyclic Silver(I) Complex of a Bis(carbone) Pincer Ligand: Synthesis and Application as a Carbone-Transfer Agent. Inorganic Chemistry 2023-08, 62, 12613-12619.
- Lai Ting Yi, Chen Chang‐Ting, Chu Kai‐Ti, Chien Su‐Ying, Ong Tiow‐Gan*, Chiang Ming‐Hsi* Biologically inspired <scp>3Fe4S</scp> cluster as structural mimics of <scp>FeMoco</scp> M‐cluster. Journal of the Chinese Chemical Society 2023-03, 70(5), 1029-1037.
- Cheng-Han Yu, Chen-Hsun Hung, Ting-Hsuan Wang, Tiow-Gan Ong* Selective C–H activation of pyridine via Ni–Al. Trends in Chemistry 2022-12, In press.
- Azina Rahmani, Taylor M. Currie, Lorianne R. Shultz, Jacob T. Bryant, Melanie J. Beazley, Fernando J. Uribe-Romo, Laurene Tetard, Nicholas G. Rudawski, Shaohua Xie, Fudong Liu, Ting-Hsuan Wang, Tiow-Gan Ong, Lei Zhai, Titel Jurca* Robust palladium catalysts on nickel foam for highly efficient hydrogenations. Catalysis Science & Technology 2022-11, 12(23), 6992-6997.
- Ting‐Hsuan Wang, Tsz‐Fai Leung, Yu‐Fu Liang, Chung‐Yu Wang, Tiow‐Gan Ong* Bis(pyridyl)carbodicarbene supported ruthenium complexes and their catalytic application in <scp>hydrogen‐transfer</scp> reaction. Journal of the Chinese Chemical Society 2022-08, 69(8), 1400-1405.
- Ming-Chun Wu, Yu-Fu Liang, Titel Jurca, Glenn P. A. Yap, Tsz-Fai Leung*, and Tiow-Gan Ong* Reactive Dicarbon as a Flexible Ligand for Transition-Metal Coordination and Catalysis. JOURNAL OF THE AMERICAN CHEMICAL SOCIETY 2022-07-07, 144, 12996-13005.
- Aweke Bamlaku Semagne, Yu Cheng‐Han, Zhi Minna, Chen Wen‐Ching, Yap Glenn P. A., Zhao Lili*, Ong Tiow‐Gan* A <i>Bis</i> ‐(carbone) Pincer Ligand and Its Coordinative Behavior toward Multi‐Metallic Configurations. Angewandte Chemie International Edition 2022-06-13, 61, e202201884.
- He Lipeng, Hsu Hung-Kai, Li Lijie, Lin Lin-Ting, Tu Tsung-Han, Ong Tiow-Gan, Liou Gunn-Guang*, Chan Yi-Tsu* A 10-nm-sized multicompartment cuboctahedron and its 2D hierarchical arrays observed by cryo-EM. Chem 2022-02-10, .
- Hsuan Chang, Wen-Ching Chen, Jiun-Shian Shen, Tiow-Gan Ong, Vincent C.-C. Wang*, Glenn P. A. Yap* Mirror-plane disorder in a nickel chloride Schiff base complex: a suitable case study for crystallographic instruction. Acta Crystallographica Section C Structural Chemistry 2022-02-08, 78(3), 137-140.
- Chan Yi-Chen, Bai Yuna, Chen Wen-Ching, Chen Hsing-Yin, Li Chen-Yu, Wu Ying-Yann, Tseng Mei-Chun, Yap Glenn P. A., Zhao Lili*, Chen Hsuan-Yin*, Ong Tiow-Gan* Synergistic Catalysis via Brønsted Acid Modulated Frustrated Lewis Pair‐Like Reaction in Carbodicarbene19949-19956. ANGEWANDTE CHEMIE-INTERNATIONAL EDITION 2021-09-01, 60, 19949-19956.
- Leung Tsz-Fai, Jiang Dandan, Wu Ming-Chun, Xiao Dengmengfei, Ching Wei-Min, Yap Glenn P. A., Yang Tao, Zhao Lili*, Ong Tiow-Gan*, Frenking Gernot* Isolable dicarbon stabilized by a single phosphine ligand. Nature Chemistry 2021, 13(1), 89-93.
- Liu Shu-kai, Chen Wen-Ching, Yap Glenn P. A., Ong Tiow-Gan* Synthesis of Carbophosphinocarbene and Their Donating Ability: Expansion of the Carbone Class. Organometallics 2020, 39(23) 4395-4401.
- Ambre Ram, Wang Ting‐Hsuan, Xian Anmei, Chen Yu‐Shiuan, Liang Yu‐Fu, Jurca Titel*, Zhao Lili*, Ong Tiow‐Gan* Directing Group‐Promoted Inert C−O Bond Activation Using Versatile Boronic Acid as a Coupling Agent. Chemistry – A European Journal 2020, 26(71) 17021-17026.
- Au‐Yeung Ka‐Chun, Xiao Dengmengfei, Shih Wei‐Chih, Yang Hsiu‐Wen, Wen Yuh‐Sheng, Yap Glenn P. A., Chen Wen‐Ching, Zhao Lili*, Ong Tiow‐Gan* Carbodicarbene: geminal ‐Bimetallic Coordination in Selective Manner. Chemistry – A European Journal 2020, 26(72) 17350-17355.
- Ram Ambre, Hsuan Yang, Wen-Ching Chen, Glenn Yap, Titel Jurca, Tiow-Gan Ong* Nickel Carbodicarbene Catalyzes Kumada Cross-Coupling of Aryl Ethers with Grignard Reagents through C-O Bond Activation. EUROPEAN JOURNAL OF INORGANIC CHEMISTRY 2019-08-31, 2019, 3511-3517.
- Patlolla Shashank Reddy, Kao Chen-Rui, Chen Guan-Wei, Huang Yu-Cheng, Chuang Yu-Chun, Sneed Brian T., Chou Wu-Ching, Ong Tiow-Gan,* Dong Chung-Li, Kuo Chun-Hong* Au-BINOL Hybrid Nanocatalysts: Insights into the Structure-Based Enhancement of Catalytic and Photocatalytic Performance. Industrial & Engineering Chemistry Research 2019, 58(14) 5479-5489.
- Wang Ting-Hsuan, Ambre Ram, Wang Qing, Lee Wei-Chih, Wang Pen-Cheng, Liu Yuhua, Zhao Lili*, Ong Tiow-Gan* Nickel-Catalyzed Heteroarenes Cross Coupling via Tandem C–H/C–O Activation. ACS Catalysis 2018-12, 11368-11376.
- Patlolla Shashank Reddy, Kao Chen-Rui, Yeh Ai-Hsuan, Lin Hung-Min, Chuang Yu-Chun, Wen Yuh-Sheng, Sneed Brian T., Chen Wen-Ching, Ong Tiow-Gan*, Kuo Chun-Hong* Interface-Controlled Synthesis of Au-BINOL Hybrid Nanostructures and Mechanism Study. Langmuir 2018-10, 34(45) 13697-13704.
- Hsu Yu-Cheng, Wang Vincent C.-C., Au-Yeung Ka-Chun, Tsai Chung-Yu, Chang Chun-Chi, Lin Bo-Chao, Chan Yi-Tsu, Hsu Chao-Ping, Yap Glenn P. A, Jurca Titel*, Ong Tiow-Gan* One‐Pot Tandem Photoredox and Cross‐Coupling Catalysis with a Single Palladium Carbodicarbene Complex. Angewandte Chemie International Edition 2018-04-16, 57(17), 4622-4626.
- Liu Shu-kai, Shih Wei-Chih, Chen Wen-Ching, Ong Tiow-Gan* Carbodicarbenes and their Captodative Behavior in Catalysis. ChemCatChem 2018-03-15, .
- Wen-Ching Chen, Wei-Chih Shih, Titel Jurca, Lili Zhao, Diego M. Andrada, Chun-Jung Peng, Chun-Chi Chang, Shu-kai Liu, Yi-Ping Wang, Yuh-Sheng Wen, Glenn P. A. Yap, Chao-Ping Hsu, Gernot Frenking, Tiow-Gan Ong* Carbodicarbenes: Unexpected π-Accepting Ability during Reactivity with Small Molecules. JOURNAL OF THE AMERICAN CHEMICAL SOCIETY 2017-12-31, 139, 12830-12836.
- Wei-Chih Shih, Yun-Ting Chiang, Qing Wang, Ming-Chun Wu, Glenn P. A. Yap, Lili Zhao*, Tiow-Gan Ong* Invisible Chelating Effect Exhibited between Carbodicarbene and Phosphine through π–π Interaction and Implication in the Cross-Coupling Reaction. Organometallics 2017, 36(21), 4287-4297.
- Ting-Hsuan Wang, Wen-Ching Chen*, Tiow-Gan Ong* Carbodicarbenes or Bent Allenes. Journal of the Chinese Chemical Society 2017, 64(2), 124-132.
- Wang Ting-Hsuan, Lee Wei-Chih, Ong Tiow-Gan* Ruthenium-Mediated Dual Catalytic Reactions of IsoquinolineviaC−H Activation and Dearomatization for Isoquinolone. Advanced Synthesis & Catalysis 2016, 358(17), 2751-2758.
- Jurca Titel, Ouanounou Sarah, Shih Wei-Chih, Ong Tiow-Gan, Yap Glenn P. A., Korobkov Ilia, Gorelsky Serge*, Richeson Darrin* Structural and electronic trends for five coordinate 1st row transition metal complexes: Mn(ii) to Zn(ii) captured in a bis(iminopyridine) framework. Dalton Transactions 2016, 45(36), 14327-14334.
- Cheng-Yuan Liu, Ying-Ho Chen, Chih-Hao Chen, Ming-Shiuan Yu, Fu-Yu Tsai*, Tiow-Gan Ong* Selective C(8)–H Activation of Imidazopyridines Mediated by Cooperative Nickel–Aluminum Catalysis. Synthesis 2016, 48(17), 2781-2788.
- Wen-Ching Chen, Jiun-Shian Shen, Titel Jurca, Chun-Jung Peng, Yen-Hsu Lin, Yi-Ping Wang, Wei-Chih Shih, Glenn P. A. Yap, Tiow-Gan Ong* Expanding the Ligand Framework Diversity of Carbodicarbenes and Direct Detection of Boron Activation in the Methylation of Amines with CO2. Angewandte Chemie International Edition 2015-12, 54, 15207-15212.
- Wei-Chih Lee, Chien-Hung Chen, Cheng-Yuan Liu, Ming-Shiuan Yu, Yung-Huei Lin, Tiow-Gan Ong* Nickel-catalysed para-CH activation of pyridine with switchable regioselective hydroheteroarylation of allylarenes . CHEMICAL COMMUNICATIONS 2015-09, 51, 17104-17107.
- Wei-Chih Lee, Wei-Chin Shih, Ting-Hsuan Wang, Yuhua Liu, Glenn P.A. Yap, Tiow-Gan Ong* Nickel promoted switchable hydroheteroarylation of cyclodienes via C–H bond activation of heteroarenes. TETRAHEDRON 2015-07, 71, 4460-4464.
- Yu-Chen Hsu, Jiun-Shian Shen, Bo-Chao Lin, Wen-Ching Chen, Yi-Tsu Chan, Wei-Min Ching, Glenn P. A. Yap, Chao-Ping Hsu,* Tiow-Gan Ong* Isolation of Tridentate Acyclic Bis(Pyridine)carbodicarbene and Further Studies on Its Structural Implications and Reactivities. ANGEWANDTE CHEMIE-INTERNATIONAL EDITION 2015-02, 54, 2420–2424.
- Ming-Shiuan Yu, Wei-Chih Lee, Chih-Hao Chen, Fu-Yu Tsai and Tiow-Gan Ong† Controlled Regiodivergent C-H Bond Activation of Imid-azo[1,5-a]pyridine via Synergistic Cooperation between Alu-minum and Nickel. ORGANIC LETTERS 2014-09, 16, 4826-4829..
- Chia-Cheng Tai, Ming-Shiuan Yu, Yi-Lin Chen, Wen-Hang Chuang, Ting-Hua Lin, Glenn P. A. Yap, Tiow-Gan Ong* Synthesis of a guanidine NHC complex and its application in borylation reactions . CHEMICAL COMMUNICATIONS 2014-03, 50, 4344-4346.
- Wen-Ching Chen, Ying-Chieh Lai, Wei-Chun Shih, Ming-Shiuan Yu, Glenn P. A. Yap,Tiow-Gan Ong* Mechanistic Study of the Regioselective Switch for Hydroheteroarylation of Styrene Catalyzed by Bimetallic Ni-Al via C-H Activation. CHEMISTRY-A EUROPEAN JOURNAL 2014-02, 20, 8099–8105.
- Hsuan-Jui Huang, Wei-Chih Lee, Glenn P. A. Yap, Tiow-Gan Ong* Synthesis and Characterization of Amino-NHC Coinage Metal Complexes and Application for C-H Activation of Caffeine . JOURNAL OF ORGANOMETALLIC CHEMISTRY 2014-01, 761, 64–73..
- Wen-Ching Chen, Ching-Yu Lee, Bo-Chao Lin, Yu-Chen Hsu, Jiun-Shian Shen, Chao-Ping Hsu, Glenn P. A. Yap, and Tiow-Gan Ong* The Elusive Three-Coordinate Dicationic Hydrido Boron Complex. JOURNAL OF THE AMERICAN CHEMICAL SOCIETY 2014-01, 136(3), 914–917.
- Wei-Chih Lee, Ting-Hsuan Wang, Tiow-Gan Ong* Ligand Promoted Pd-Catalyzed Dehydrogenative Alkenylation of Hetereoarenes . CHEMICAL COMMUNICATIONS 2013-12, 50, 3671-3673.
- Wei-Chih Lee, Chun-Han Wang, Yung-Huei Lin, Wei-Chun Shih, Tiow-Gan Ong* Tandem Isomerization and C–H Activation: Regioselective Hydroheteroarylation of Allylarenes. ORGANIC LETTERS 2013-10, 15 (20), 5358–5361.
- Wen-Ching Chen, Yu-Chen Hsu, Ching-Yu Lee, Glenn P. A. Yap, Tiow-Gan Ong* Synthetic Modification of Acyclic Bent Allenes (Carbodicarbenes) and Further Studies on Their Structural Implications and Reactivities. ORGANOMETALLICS 2013-04, 32, 2435–2442.
- Chien-Tien Chen*, Tiow-Gan Ong Complementary Helicity Interchange of Optically Switchable Supramolecular-Enantiomeric Helicenes with (-)-Gel-Sol-(+)-Gel Transition Ternary Logic. JOURNAL OF THE AMERICAN CHEMICAL SOCIETY 2013-04, 135 (14), 5294–5297.
- Titel Jurca, Wen-Ching Chen, Sheila Michel, Ilia Korobkov, Tiow-Gan Ong, Darrin S. Richeson* Solid-State Thermolysis of a fac-Rhenium(I) Carbonyl Complex with a Redox Non-Innocent Pincer Ligand. CHEMISTRY-A EUROPEAN JOURNAL 2013-03, 19, 4278–4286.
- Wen-Ching Chen, Yu-Chen Hsu, Wei-Chun Shih, Ching-Yu Lee, Wen-Han Chuang, Yi-Fang Tsai, Peter Ping-Yu Chen*, Tiow-Gan Ong* Metal-Free Arylation of Benzene and Pyridine Promoted by Amino-Linked Nitrogen Heterocyclic Carbenes. CHEMICAL COMMUNICATIONS 2012-05, 48, 6702-6704.
- Ya-Ming Liu, Yi-Chun Lin, Wen-Ching Chen, Jen-Hao Cheng, Yi-Lin Chen, Glenn P. A. Yap, Shih-Sheng Sun*, Tiow-Gan Ong* Synthesis and characterization of para-pyridine linked NHC palladium complexes and their studies for the Heck–Mizoroki coupling reaction. DALTON TRANSACTIONS 2012-04, 41, 7382-7389.
- Wei-Chun Shih, Wen-Ching Chen, Ying-Chieh Lai, Ming-Shiuan Yu, Jhao-Jhe Ho, Glenn P. A. Yap, Tiow-Gan Ong* The Regioselective Switch for Amino-NHC Mediated C–H Activation of Benzimidazole via Ni–Al Synergistic Catalysis. ORGANIC LETTERS 2012-04, 14(8), 2046–2049.
- Chia-Cheng Tai, Ya-Ting Chang, Jie-Hong Tsai, Titel Jurca, Glenn P. A. Yap, Tiow-Gan Ong* Subtle Reactivities of Boron and Aluminum Complexes with Amino-Linked N-Heterocyclic Carbene Ligation. ORGANOMETALLICS 2012-01, 31(2), 637–643.
- Chia-Yi Li, Yi-Yin Kuo, Jie-Hong Tsai, Glenn P. A. Yap, Tiow-Gan Ong* Amine-Linked N-Heterocyclic Carbenes: The Importance of an Pendant Free-Amine Auxiliary in Assisting the Catalytic Reaction. CHEMISTRY-AN ASIAN JOURNAL 2011-06, 6(6),1520–1524.
- Chie-Hong Wang, Wei-Chih Shih, Hui Chuan Chang, Yi-Yin Kuo, Wen-Chun Hung, Tiow-Gan Ong*, and Wen-Shan Li* Preparation and Characterization of Amino-Linked Heterocyclic Carbene Palladium, Gold, and Silver Complexes and Their Use as Anticancer Agents That Act by Triggering Apoptotic Cell Death. JOURNAL OF MEDICINAL CHEMISTRY 2011-06, 54 (14), 5245–5249.
- Jie-Hong Tsai, Shen-Ta Lin, Richard Bing-Gong Yang, Glenn P. A. Yap, and Tiow-Gan Ong Organometallics, 2010, 29 18, pp 4004–4006 Publication Date Web: August 25, 2010 Communication DOI: 10.1021/om100747j CASSJie-Hong Tsai, Shen-Ta Lin, Richard Bing- Two-Way Street Transformation of Boronium and Borane Complexes Facilitated by Amino-Linked N-Heterocyclic Carbene. ORGANOMETALLICS 2010-08, 29 (18), 4004–4006.
- Chung-Chih Tsai, Wei-Chih Shih, Cyong-Hui Fang, Chia-Yi Li, Tiow-Gan Ong*, Glenn P. A. Yap. Bimetallic Nickel Aluminun Mediated Para-Selective Alkenylation of Pyridine: Direct Observation of η2,η1-Pyridine Ni(0)−Al(III) Intermediates Prior to C−H Bond Activation.. JOURNAL OF THE AMERICAN CHEMICAL SOCIETY 2010-08, 132,11887-11889.
- Yu-Cheng Hu, Cheng-Feng Liang, Jie-Hong, Tsai, Glenn P. A. Yap, Ya-Ting Chang, Tiow-Gan Ong* Zirconium Complexes Supported by Imidazolones: Synthesis, Characterization, and Application of Precatalysts for the Hydroamination of Aminoalkenes.. ORGANOMETALLICS 2010-07, 29(15), 3357-3361.
- Yu-Cheng Hu, Chung-Chih Tsai, Wei-Chih Shih, Glenn P. A. Yap, Tiow-Gan Ong* The Zirconium Benzyl Mediated C-N Bond Cleavage of an Amino-Linked N-Heterocyclic Carbene.. Organometallics 2010-01, 29(3),516-518.
- Yi-Ping Huang, Chung-Chih Tsai, Wei-Chih Shih, Yu-Chang Chang, Shen-Ta Lin, Glenn P. A. Yap, Ito Chao, Tiow-Gan Ong* Kinetic and Thermodynamic Study of Syn−Anti Isomerization of Nickel Complexes Bearing Amino-Linked N-Heterocyclic Carbene Ligands: The Effect of the Pendant Arm of the NHC.. Organometallics 2009-07, 28(15), 4316–4323.
- Wei-Chih Shih, Chun-Han Wang, Yang-Ting Chang, Glenn P. A. Yap and Tiow-Gan Ong* Synthesis and Structure of an Amino-Linked N-Heterocyclic Carbene and the Reactivity of its Aluminum Adduct. ORGANOMETALLICS 2009-01, 28(4), 1060–1067.
- Lavoie N, Ong TG*, Gorelsky SI, Korobkov I, Yap GPA, Richeson DS. Bis(imido) W(VI) complexes chelated by N,N '-Disubstituted 1,8-diamidonaphthalene: An analysis of bonding, isocyanate insertion, and Al-Me transfer. Organometallics 2007-12, 26(26), 6586-6590.
- Ong, TG, O'Brien, JS, Korobkov, I and Richeson, DS Facile and atom-efficient amidolithium-catalyzed C-C and C-N formation for the construction of substituted guanidines and propiolamidines. ORGANOMETALLICS 2006-09, 25(20), 4728-4730.
- Said, FF, Ong, TG, Bazinet, P, Yap, GPA and Richeson, DS Linking hydrogen dicarboxylate synthons with substituted guanidinium cations: Transforming rings and chains into two- and three-dimensional structures. CRYSTAL GROWTH & DESIGN 2006-08, 6(8), 1848-1857.
- Said, FF, Bazinet, P, Ong, TG, Yap, GPA and Richeson, DS Hydrogen bonding motifs of N,N ',N ''-trisubstituted guanidinium cations with spherical and rodlike monoanions: Syntheses and structures of I-, I-3(-), and SCN- salts. CRYSTAL GROWTH & DESIGN 2006-01, 6(1), 258-266.
- Tiow-Gan, Ong*; Wei-Chun, Shih; Wen-Ching, Chen, Ying-Cheih, Lai and Ming-Shiuan, Yu. , 2013, “The Odyssey of the N-Heterocyclic Carbene in Promoting C-H Functionalization”, paper presented at The 93 Annual Meeting of the Chemical Society of Japan/Asian International Symposium for Outstanding Young Scientists, Shiga, Japan: The Chemical Society of Japan, 2013-03-22 ~ 2013-03-25.
- Ya-Ming Liu; Shih-Sheng Sun; Tiow-Gan Ong*, 2010, “Synthesis and Characterization of Ag(I), Cu(I), Pd(II) and Ni(II) Complexes Supported by 4-Pyridyl-Subtituted N-Heterocyclic Carbene.”, paper presented at 24th International Conference on Organometallics Chemistry, Taipei, Taiwan: Chemical Society of Taiwan and ICOMC, 2010-07-18 ~ 2010-07-23.
- Tiow-Gan Ong*, 2010, ““The Employment of an Anionic-Nitrogen Heterocyclic Carbene in Main and Early Transition Metals for Unusual Reactivities and Possible Catalysis Application.””, paper presented at BTI’s 1st Annual World Congress of Catalytic Asymmetric Synthesis, Beijing, China: BTI, 2010-05-19 ~ 2010-05-21.
- tiow gan ong, 2009, ““Synthesis of Nickel Complexes Bearing Amino-Linked N-Heterocyclic Carbene Ligands: Syn-anti Isomerization and Its Possible Implication toward Suzuki Cross-Coupling.”, paper presented at IUPAC International Symposium on Organometallic Chemistry Directed Towards Organic Synthesis, Glasgow, Scotland, UK: IUPAC, 2009-07-26 ~ 2009-07-31.
- Tiow-Gan Ong, 2008, “Bis(imido) W(VI) Complexes Chelated by Bidentate Amide: An Analysis of Bonding, Isocyanate Insertion, and Al-Me Transfer.”, paper presented at International Conference on Organometallic Chemistry, Rennes, France: International Organometallics Chemistry, 2008-07-13 ~ 2008-07-18.
- Tiow-Gan Ong, Nathalie Lavoie, Serge Gorelsky, Illia Korobkov, Glenn Yap, Darrin Richeson, 2008, “Bis(imido)W(VI) Compleces Chelated by Bidentate Amide: An Analysis of Bonding, Isocyanate Insertion, and Al-Me Transfer”, P704 pages, paper presented at International Conference on Organometallic Chemistry, Rennes, France: University of Rennes, 2008-07-13 ~ 2008-07-18.
- Tiow-Gan ong, 2008, “Molecular Manipulation of Late Transition Group Metal Using an Amino”, paper presented at 20th Canadian Symposium on Catalysis, Kingston, Canada: Canadian Chemical Society catalsis division, 2008-06-15 ~ 2008-06-18.
- tiow-Gan ong, 2008, “Molecular Manipulation of Main and Transition Group Metal Using an Amino Pendant Linked Nitrogen Heterocyclic Carbene and Their Possible Application.”, paper presented at 91st Canadian Chemical Society Conference, Edmonton, Canada: Canadian Chemical Society, 2008-05-24 ~ 2008-05-28.
- Tiow-Gan Ong, 2007, “Bis(imido) W(VI) Complexes Chelated by N,N-Disubstituted 1,8-Diamidonaphthalene: An Analysis of Bonding, Isocyanate Insertion, and Al-Me Transfer.”, paper presented at International Symposium on Catalysis and Fine Chemicals, Singapore: Nanyang Tech University, 2007-12-17 ~ 2007-12-21.
- tiow-gan ong, 2006, “Facile and Atom Efficient Catalytic C-C and C-N Formation Using Bifunctional Main Group Catalysts.”, paper presented at Sixth Tateshina Conference on Organic Chemistry, Chino, Japan: JST, 2006-11-10.
- tiow-gan ong, 2006, “Facile and Atom Efficient Catalytic C-C and C-N Formation Using Bifunctional Main Group Catalysts.”, paper presented at Second Asian Symposium on Advanced Organic Synthesis., Kyoto Japan: Kyoto University, 2006-11-07 ~ 2006-11-09.


