Institute of Chemistry, Academia Sinica – People

Over the years, we are endeavoring on the functional studies of metalloproteins towards understanding the underlying fundamental mechanisms of high-valent metal active sites for the applications of the controlled and selective C-H bond oxidation of variable hydrocarbons including methane, natural gas, linear and branched alkanes, simple aromatics including BTX (Benzene, Toluene and Xylene) and poly-aromatics hydrocarbons (PAH) in the production of future fuels/commodity chemicals. Our results have further elucidated how these metalloproteins can perform the sustainable bioremediation processes of polluted chemicals in the prokaryotic bacteria. These outcomes are expected to have long-term impacts in environmental issues, particularly in the areas of alternative green energy, waste water treatments and soil recovery.
- Metallo-protein engineering in bio-catalysis and bio-sensing
The major aim of our research work is to explore various ways to achieve regio-selective and stereo-selective oxidations of hydrocarbons including aliphatics (normal alkanes) and aromatics under ambient conditions with high efficiency and atom economy. When researchers seek to account for differences in the chemical selectivity of metallo-enzymes or monooxygenases, they typically invoke differences in shape selection of substrate pockets as well as differences in specific non-bonding interactions, such as hydrogen bonding, electrostatic forces, and/or van der Waals interactions, in the interactions of the hydrocarbon substrates with the active sites of the metalloenzymes/monooxygenases. (Chem. Eur. J. 2011&2013, Tetrahedron Lett. 2011 and J. Inorg. Biochem. 2014) To explore these effects in depth, we have redesigned the substrate binding pockets of alkane hydroxylase (AlkB) and cytochrome P450 BM3 using a heterogeneous Escherichia coli recombinant system. (Chem. Eur. J. 2017 and Sci. Reports, 2017) These two redesigned proteins are deployed to achieve efficient oxidations of n-butane at its C-1 and C-2 positions, respectively. These studies have enabled us to propose mechanisms used by AlkB and cytochrome P450 BM3 to selectively oxidize inert C-H bonds and oxyfunctionalize a variety of hydrocarbons. The insights gained are expected to contribute to the design of biomimetics or artificial catalysts that selectively oxidize hydrocarbons, including nanomaterial platforms of these biomimetics.
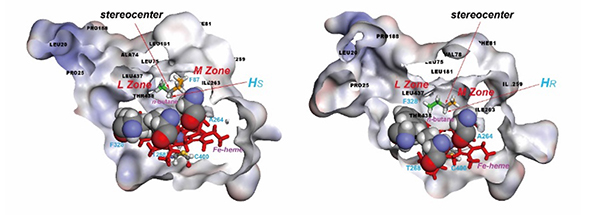
Selective secondary C–H bond oxidation of n-butane mediated by engineered recombinant cytochrome P450 BM3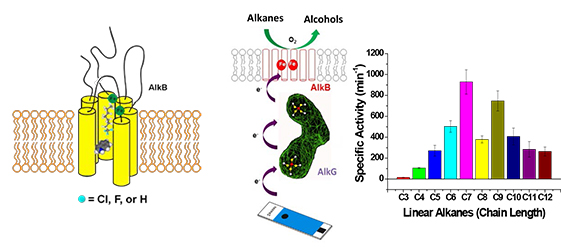
Selective primary C-H bond oxidation of C3-C12 linear alkanes mediated by the engineered recombinant alkane hydroxylase (AlkB) from Pseudomonas putida GPo1Superoxide responsive protein (SoxR), a transcriptional factor, is a homodimeric protein with each subunit containing a redox-active [2Fe-2S] center. The iron–sulfur clusters within SoxR regulate transcriptional activity in response to one-electron oxidation, from the paramagnetic mixed-valence [FeII-FeIII] to the diamagnetic [FeIII-FeIII] species; a process that is presumably mediated by oxidative stress. The corresponding oxidation could trigger the expression of the soxS gene and subsequently activate numerous defensive genes, such as superoxide dismutase or catalase in Escherichia coli. In the case of nitrosylated SoxR, the results on about 2-fold spin quantitation relative to the model compounds of low molecular weight-dinitrosyl iron complex (LMW-DNIC) at low temperture EPR spectroscopy, the absence of dichoric spectral features in the responsive area of the nitrosyl iron complexes as well as the lack of Fe-Fe backscattering resolved by EXAFS fitting have strongly supported that the situation appears to be dramatically different that each of the [2Fe-2S] cores in SoxR is dissociated into two individual dinitrosyl iron complexes after nitrosylation. We have recently demonstrated that the formation of DNICs from Fe–S clusters leads to rich chemistry, including the formation of a reduced Roussin's red ester (rRRE) and a diamagnetic species of anionic Roussin's red ester (RRE), which both are redox sensitive. Accordingly, under nitrosative stresses, it is conceivable that the nitrosylated SoxR could also sense oxidative stresses from the cytoplasmic diffusible oxidants, DNA lesion or damage. (Chem. Eur. J. 2012)
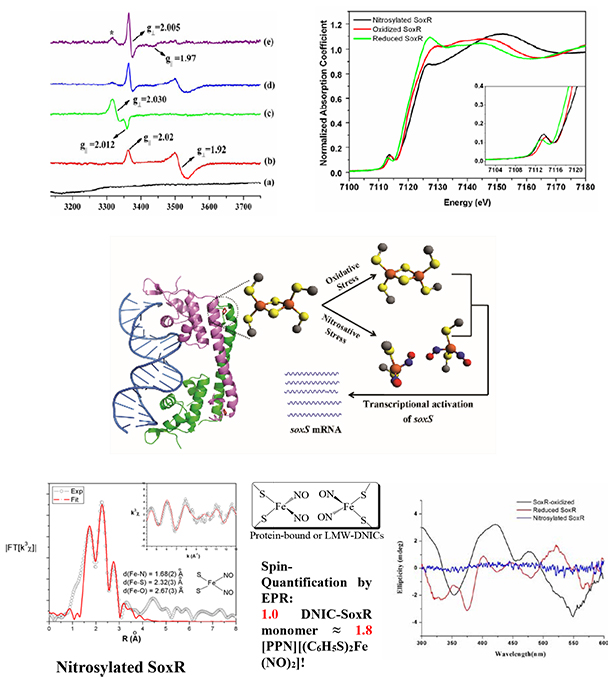
X-ray absorption (XAS), circular dichroism (CD) and electron paramagnetic resonance (EPR) spectroscopic studies of SoxR protein from Escherichia coli - Nano bio-mimetic system for the selective oxidation in hydrocarbon
Yu laboratory recently discovers a series of Fe/Cu based organic-inorganic hybrid nanoparticle catalysts that can be prepared by their metal salts using H2O2(aq) in CH3CN. (Mol. Catal. 2017 and J. Catal. 2019) These nano-catalysts efficiently conduct the C–H bond activations of benzene to form phenol and/or p-benzoquinone, and of toluene to form benzaldehyde, benzyl alcohol, o-cresol, p-cresol, and/or methyl-p-benzoquinone. These reactions can be facilely tuned and controlled to selectively yield either a single or double oxygenation of benzene as well as a sp3 or sp2 C–H bond oxidation of toluene.
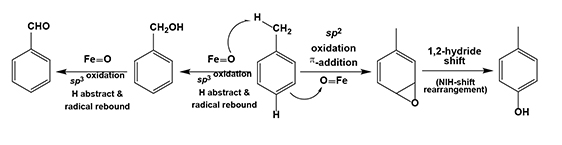
Chain or ring oxidations of toluene by high-valence metal oxygenated species from the active sites of metalloproteins, i.e., cytochrome P450s.
In future, Yu laboratory will direct their research attention towards the research topics of “catalysis” in oxy-functionalization of inert hydrocarbons to develop the metal based monooxygenases/metal oxide nanomaterials catalysts with the metal cluster features that can mimic bacterial monooxygenases for a sustainable oxidation of hydrocarbons by the employment of electrochemical means driven by the renewable energy such as solar and wind power. (Sci. Reports 2017 and Angew. Chem. Int. Ed. 2018) The main research topics will be the controlled partial oxidation of methane, small alkanes and simple aromatics for commodity chemical/fuel production.
- "Chinese Chemical Society Annual Best Article Award 中國化學會最佳論文獎" (2005-12)
- Wanna, W. H.; Ramu, R.; Janmanchi, D.; Tsai, Y.-F.; Thiyagarajan, N.; Yu, S. S.-F. An Efficient and Recyclable Copper Nano-catalyst for the Selective Oxidation of Benzene to p-Benzoquinone (p-BQ) using H2O2(aq) in CH3CN. J. Catal. 2019, accepted.
- Thiyagarajan, N.; Janmanchi, D.; Tsai, Y.-F.; Wanna, W. H.; Ramu, R.; Chan, S. I.*; Zen, J.-M.;*, Yu, S. S.-F.* A Carbon Electrode Functionalized by a Tricopper Cluster Complex: Overcoming Overpotential and Production of Hydrogen Peroxide in the Oxygen Reduction Reaction. Angew. Chem.-Int. Ed., 2018, 57, 3612.
- Yang, C.-L.; Lin, C.-H.; Luo W.-I.; Lee, T.-L.; Ramu, R.; Ng, K. Y.; Tsai, Y. F.; Wei, G.-T.; Yu, S. S.-F.* Mechanistic Study for the Stereoselective Hydroxylation of [2-²H₁,3-²H₁]Butanes Catalyzed by Cytochrome P450 BM3 Variants. Chem. Eur. J. 2017, 23, 2571.
- Tsai, Y.-F.; Luo, W.-I.; Chang, J.-L; Chang, C.-W.; Ramu, R.; Choung, H.-C.; Wei, G.-T.; Zen, J.-M.*; Yu, S. S.-F.* Electrochemical Hydroxylation of C3-C12 n-Alkanes by a Recombinant Alkane Hydroxylase (AlkB) and its Redox Partner, Rubredoxin-2 (AlkG), from Pseudomonas putida GPo1. Sci. Reports 2017, 7(1), DOI: 10.1038/s41598-017-08610-w.
- Ramu, R.; Wanna W. H.; Janmanchi, D.; Tsai, Y.-F.; Liu, C.-C.; Mou, C.-Y.; Yu, S. S.-F.* Selective Oxidation of Benzene and Toluene by Fe(ClO4)2 in an H2O2-H2O-CH3CN system. Mol. Catal. 2017, 441, 114.
- Chen, Y.-S.; Luo, W.-I.; Yang C.-L.; Tu, Y.-J.; Chang C.-W.; Chiang, C.-H.;Chang, C.-Y.; Chan, S. I.; Yu, S. S.-F.* Controlled Oxidation of Aliphatic C-H Bonds in Metallo-monooxygenases: Mechanistic Insights Derived from Studies on Deuterated and Fluorinated Hydrocarbons. J. Inorg. Biochem. 2014, 134, 118. (Early Career Focused Review, Cover Art)
- Chiang, C.-H.; Ramu, R.; Tu, Y.-J.; Yang, C.-L.; Ng, K. Y.; Luo, W.-I; Chen, C. H.; Lu, Y.-Y.; Liu, C.-L.; Yu, S. S.-F.* Regio-Selective Hydroxylation of C12-C15 Fatty Acids with Fluorine Substituents by Cytochrome P450 BM3. Chem.-Eur. J. 2013, 19, 13680. (highlighted as a frontispiece of the issue)
- Chen, Y.-S.; Luo W.-I.; Lee, T.-L.; Yu, S. S.-F.*; Chang, C.-Y.* Identification of the Proteins Required for Fatty Acid Desaturation in Zebrafish (Danio rerio). Biochem. Biophys. Res. Commun. 2013, 440, 671.
- Lo, F.-C.; Lee, J.-F.; Liaw, W.-F.; Hsu, I-J.*; Chan, S. I.; Tsai, Y.-F.; Yu, S. S.-F.* The Metal Core Structures in the Recombinant Escherichia coli Transcriptional Factor SoxR. Chem.-Eur. J. 2012, 18, 2565.
- Wu, L.-L.; Yang, C.-L.; Lo, F.-C.; Chiang, C.-H.; Chang, C.-W.; Ng, K. Y.; Chou, H.-H.; Hung, H.-Y.; Chan, S. I.; Yu, S. S.-F.* Tuning the Regioselectivity and Stereoselectivity in C-H Activation of n-Octanes by Cytochrome P450 BM-3 with Fluorine Substituents. Evidence for Interactions between a C-F Bond with Aromatic pi-Systems. Chem. Eur. J. 2011, 17, 4774.
- Ramu, R.; Chang, C.-W.; Chou, H.-H.; Wu, L.-L.; Chiang, C.-H.; Yu, S. S.-F.* Regio-selective Hydroxylation of gem-Difluorinated Octanes by Alkane Hydroxylase (AlkB). Tetrahedron Lett. 2011, 52(23), 2950.
Update: 2019-01-07
- Nigussie Gebretinsae Yeabyo, Tsai Yi-Fang, Yang Tsung-Cheng, Yang Chia-Min, Yu Steve S.-F. An efficient H<sub>2</sub>O<sub>2</sub>-based propylene to propylene oxide (HPPO) reaction catalyzed by ZnO/ZnO<sub>2</sub> materials. Journal of Materials Chemistry A 2025, 13(7), 5261-5274.
- Zarour Ahmad, Omar Suheir, Yu Steve S.-F., Abu-Reziq Raed* Highly efficient and selective hydroformylation by rhodium-based PEG@silica hybrid microreactors. Molecular Catalysis 2025, 572, 114807.
- Cabanding Jaidriel Meg G., Yu Steve S.-F., Lin Zhi-Han, Fortuna Myrnel A., Elatico Adam Jo J., Nellas Ricky B.* The importance of helical structures to the overall activity and structural stability of a lipase from Pseudomonas aeruginosa PAO1 in n-hexane. Archives of Biochemistry and Biophysics 2025, 764, 110226.
- Fortuna Myrnel A., Cabanding Jaidriel Meg G., Yu Steve S.-F., Lin Zhi-Han, Elatico Adam Jo J., Nellas Ricky B.* Computational reverse protein engineering of a lipase from Pseudomonas aeruginosa in n-hexane. Computational and Structural Biotechnology Reports 2024, 1, 100025.
- Chin Hong Soon, Ravi Varadharajulu Narendrakumar, Lin Zhi-Han, Hsu Chuan-Chih, Yu Steve S.-F.* Phosphoproteomic analysis reveals distinctive responses in Mangrovibacter phragmatis under high-salinity condition. Biochemical and Biophysical Research Communications 2024, 736, 150514.
- Thiyagarajan Natarajana, Sankar Arumugama, Yi-Fang Tsai, Asia Abou-taleb, Steve S.-F. Yu* Unveiling the enhanced electrochemical CO2 conversion: The role of 3D porous BiOCl with defects and CTAB-mediated nanosheets. JOURNAL OF CO2 UTILIZATION 2024, 85, 102888.
- Hong Soon Chin, Narendrakumar Ravi Varadharajulu, Zhi-Han Lin, Wen-Yu Chen, Zong-Han Zhang, Sankar Arumugam, Ching-Yen Lai, Steve S.-F. Yu* Isolation, molecular identification, and genomic analysis of Mangrovibacter phragmitis strain ASIOC01 from activated sludge harboring the bioremediation prowess of glycerol-and organic pollutants in high-salinity. FRONTIERS IN MICROBIOLOGY 2024, 15, 1415723.
- Abay Tigist Ayalew, Wanna Wondemagegn H., Natarajan Thiyagarajan, Tsai Yi-Fang, Janmanchi Damodar, Jiang Jyh-Chiang*, Abu-Reziq Raed*, Yu Steve S.-F.* Selective oxidation of benzene by an iron oxide carbonaceous nanocatalyst prepared from iron perchlorate salts and hydrogen peroxide in benzene and acetonitrile. Molecular Catalysis 2022-06-01, 526, 112397.
- Yi-Fang Tsai, Thiyagarajan Natarajan, Zhi-Han Lin, I-Kuen Tsai, Damodar Janmanchi, Sunney I. Chan*, Steve S.-F. Yu* Voltage-Gated Electrocatalysis of Efficient and Selective Methane Oxidation by Tricopper Clusters under Ambient Conditions. Journal of the American Chemical Society 2022-05-27, 10.1021/jacs.2c01169.
- Sunney I. Chan*, Vincent C.‐C. Wang, Peter P.‐Y. Chen, Steve S.‐F. Yu* Methane oxidation by the copper methane monooxygenase: Before and after the cryogenic electron microscopy structure of particulate methane monooxygenase from <i>Methylococcus capsulatus</i> (Bath). Journal of the Chinese Chemical Society 2022-05-23, 1-12.
- Shih Shou-Ping, Lu Mei-Chin, El-Shazly Mohamed, Lin Yu-Hsuan, Chen Chun-Lin*, Yu Steve Sheng-Fa*, Liu Yi-Chang* The Antileukemic and Anti-Prostatic Effect of Aeroplysinin-1 Is Mediated through ROS-Induced Apoptosis via NOX Activation and Inhibition of HIF-1a Activity. Life 2022, 12(5), 687.
- Lu Yu‐Jhang, Janmanchi Damodar, Natarajan Thiyagarajan, Lin Zhi‐Han, Wanna Wondemagegn H., Hsu I‐Jui, Tzou Der‐Lii M., Ayalew Abay Tigist, Yu Steve S.‐F.* Silver Cyanide Powder‐Catalyzed Selective Epoxidation of Cyclohexene and Styrene with its Surface Activation by H<sub>2</sub>O<sub>2(aq)</sub> and Assisted by CH <sub>3</sub>CN as a Non‐Innocent Solvent. ChemCatChem 2022, 14, e202200030-DOI: 10.1002/cctc.20.
- Chan Sunney I.*, Chang Wei-Hau*, Huang Shih-Hsin, Lin Hsin-Hung, Yu Steve S.-F.* Catalytic machinery of methane oxidation in particulate methane monooxygenase (pMMO). Journal of Inorganic Biochemistry 2021-12, 225, 111602.
- Wu Danni, Carillo Kathleen Joyce, Shie Jiun-Jie, Yu Steve S.-F., Tzou Der-Lii M.* Resolving Entangled JH-H-Coupling Patterns for Steroidal Structure Determinations by NMR Spectroscopy. Molecules 2021, 26(9), 2643.
- Peng Bo-Rong, Lai Kuei-Hung, Lee Gene-Hsiang, Yu Steve Sheng-Fa, Duh Chang-Yih, Su Jui-Hsin, Zheng Li-Guo, Hwang Tsong-Long*, Sung Ping-Jyun* Scalarane-Type Sesterterpenoids from the Marine Sponge Lendenfeldia sp. Alleviate Inflammation in Human Neutrophils. Marine Drugs 2021, 19(10), 561.
- Chang W.-H.*, Lin H.-H., Tsai I-K., Huang S.-H., Chung S.-C., Tu I-P., Yu S. S.-F.*, Chan S. I.* Copper Centers in the Cryo-EM Structure of Particulate Methane Monooxygenase Reveal the Catalytic Machinery of Methane Oxidation. Journal of the American Chemical Society 2021, 143(26), 9922-9932.
- Chan Sunney I.*, Chuankhayan Phimonphan, Reddy Nareddy Pavan Kumar, Tsai I-Kuen, Tsai Yi-Fang, Chen Kelvin H.-C., Yu Steve S.-F.*, Chen Chun-Jung* Mechanism of Pyrroloquinoline Quinone-Dependent Hydride Transfer Chemistry from Spectroscopic and High-Resolution X-ray Structural Studies of the Methanol Dehydrogenase from Methylococcus capsulatus (Bath). Journal of the American Chemical Society 2021, 143(9), 3359-3372.
- Wanna Wondemagegn H., Janmanchi Damodar, Thiyagarajan Natarajan, Ramu Ravirala, Tsai Yi-Fang, Yu Steve S. F. Selective Oxidation of Simple Aromatics Catalyzed by Nano-Biomimetic Metal Oxide Catalysts: A Mini Review. Frontiers in Chemistry 2020, 8, 589178.
- Peng Bo-Rong, Lai Kuei-Hung, Chen You-Ying, Su Jui-Hsin, Huang Yusheng M., Chen Yu-Hsin, Lu Mei-Chin, Yu Steve Sheng-Fa*, Duh Chang-Yih*, Sung Ping-Jyun* Probing Anti-Proliferative 24-Homoscalaranes from a Sponge Lendenfeldia sp.. Marine Drugs 2020, 18(2), 76.
- Liu Chih-Cheng, Chou Hao-Ju, Lin Chan-Yi, Janmanchi Damodar, Chung Po-Wen, Mou Chung-Yuan, Yu Steve S.-F., Chan Sunney I*. The oversolubility of methane gas in nano-confined water in nanoporous silica materials. Microporous and Mesoporous Materials 2020, 293 109793.
- Chan Sunney I.*, Yu Steve S.-F., Liu Chih-Cheng, Mou Chung-Yuan* Selective oxidation of light alkanes under mild conditions. Current Opinion in Green and Sustainable Chemistry 2020, 22 39-46.
- Peng Bo-Rong, Lai Kuei-Hung, Chang Yu-Chia, Chen You-Ying, Su Jui-Hsin, Huang Yusheng M., Chen Po-Jen, Yu Steve Sheng-Fa*, Duh Chang-Yih*, Sung Ping-Jyun* Sponge-Derived 24-Homoscalaranes as Potent Anti-Inflammatory Agents. Marine Drugs 2020, 18(9), 434.
- Wanna Wondemagegn Hailemichael, Janmanchi Damodar, Thiyagarajan Natarajan, Ramu Ravirala, Tsai Yi-Fang, Pao Chih-Wen, Yu Steve S.-F.* Selective catalytic oxidation of benzene to phenol by a vanadium oxide nanorod (Vnr) catalyst in CH3CN using H2O2(aq) and pyrazine-2-carboxylic acid (PCA). New Journal of Chemistry 2019-10, 43(45) 17819-17830.
- Chan Sunney I.*, Yu Steve S.-F Copper protein constructs for methane oxidation. Nature Catalysis 2019-04, 2(4) 286-287.
- Liu Chih-Cheng, Tsai Yi-Fang, Mou Chung-Yuan*, Yu Steve S.-F., Chan Sunney I.* Dicopper Dioxygenase Model Immobilized in Mesoporous Silica Nanoparticles for Toluene Oxidation: A Mechanism to Harness Both O Atoms of O2 for Catalysis. The Journal of Physical Chemistry C 2019-04, 123(17) 11032-11043.
- Yu-Jhang Lu, Mu-Cheng Hung, Brian T.-A. Chang, Tsu-Lin Lee, Zhi-Han Lin, I-Kuen Tsai, Yao-Sheng Chen, Chin-Shuo Chang, Yi-Fang Tsai, Kelvin H.-C. Chen, Sunney I. Chan*, Steve S.-F. Yu* The PmoB Subunit of Particulate Methane Monooxygenase (pMMO) in Methylococcus capsulatus (Bath): The CuI Sponge and its Function. JOURNAL OF INORGANIC BIOCHEMISTRY 2019-04, .
- Wanna Wondemagegn Hailemichael, Ramu Ravirala, Janmanchi Damodar, Tsai Yi-Fang, Thiyagarajan Natarajan, Yu Steve S.-F.* An efficient and recyclable copper nano-catalyst for the selective oxidation of benzene to p-benzoquinone (p-BQ) using H2O2(aq) in CH3CN. Journal of Catalysis 2019-02, 370, 332-346.
- Thiyagarajan Natarajan, Janmanchi Damodar, Tsai Yi-Fang, Wanna Wondemagegn Hailemichael, Ramu Ravirala, Chan Sunney I.*, Zen Jyh-Myng*, Yu Steve S.-F.* A Carbon Electrode Functionalized by a Tricopper Cluster Complex: Overcoming Overpotential and Production of Hydrogen Peroxide in the Oxygen Reduction Reaction. Angewandte Chemie International Edition 2018-04, 57, DOI 10.1002/anie.20171226.
- Yeh Chen-Hao, Yu Steve S.-F., Chan Sunney I., Jiang Jyh-Chiang* Quantum Chemical Studies of Methane Oxidation to Methanol on a Biomimetic Tricopper Complex: Mechanistic Insights. ChemistrySelect 2018, 3(18) 5113-5122.
- Liu Chih-Cheng, Janmanchi Damodar, Wen Da-Ren, Oung Jung-Nan, Mou Chung-Yuan, Yu Steve S.-F., Chan Sunney I. Catalytic Oxidation of Light Alkanes Mediated at Room Temperature by a Tricopper Cluster Complex Immobilized in Mesoporous Silica Nanoparticles. ACS Sustainable Chemistry & Engineering 2018, 6(4), 5431-5440.
- Ravirala Ramu, Wondemagegn Hailemichael Wanna, Damodar Janmanchi, Yi-Fang Tsai, Chih-Cheng Liu, Chung-Yuan Mou, Steve S.-F. Yu* Mechanistic study for the selective oxidation of benzene and toluene catalyzed by Fe(ClO4)2 in an H2O2 -H2O-CH3CN system. Molecular Catalysis 2017-11, 441, 114-121.
- Yi-Fang Tsai, Wen-I Luo, Jen-Lin Chang, Chun-Wei Chang, Huai-Chun Chuang, Ravirala Ramu, Guor-Tzo Wei, Jyh-Myng Zen, Steve S.-F. Yu* Electrochemical Hydroxylation of C3–C12 n-Alkanes by Recombinant Alkane Hydroxylase (AlkB) and Rubredoxin-2 (AlkG) from Pseudomonas putida GPo1. Scientific Reports 2017-08, 7(1), DOI: 10.1038/s41598-017-08610-w.
- Vincent C.-C. Wang, Suman Maji, Peter P.-Y. Chen, Hung Kay Lee, Steve S.-F. Yu, Sunney I. Chan* Alkane Oxidation: Methane Monooxygenases, Related Enzymes, and Their Biomimetics. Chemical Reviews 2017-07, 117(13), 8574-8621.
- Chung-Ling Yang, Cheng-Hung Lin, Wen-I Luo, Tsu-Lin Lee, Ravirala Ramu, Kok Yaoh Ng, Yi-Fang Tsai, Guor-Tzo Wei, Steve S.-F. Yu* Mechanistic Study of the Stereoselective Hydroxylation of [2-2H1,3-2H1]Butanes Catalyzed by Cytochrome P450 BM3 Variants. Chemistry - A European Journal 2017-02, 23(11), 2571-2582.
- Chih-Cheng Liu, Ravirala Ramu, Sunney I. Chan*, Chung-Yuan Mou*, Steve S.-F. Yu* Chemistry in confined space: a strategy for selective oxidation of hydrocarbons with high catalytic efficiencies and conversion yields under ambient conditions. Catalysis Science & Technology 2016-09, 6. 7623-7630.
- Chih-Cheng Liu, Chung-Yuan Mou, Steve S.-F. Yu, Sunney I. Chan* Heterogeneous formulation of the tricopper complex for efficient catalytic conversion of methane into methanol at ambient temperature and pressure. Energy & Environmental Science 2016-01, 9, 1361-1374.
- Minh D. Pham, Ya-Ping Lin, Quan Van Vuong, Penumaka Nagababu,m Brian T.-A. Chang, Kok Yaoh Ng, Chien-Hung Chen, Chau-Chung Han, Chung-Hsuan Chen, Mai Suan Li, Steve S.-F. Yu*, Sunney I. Chan* Inactivation of the particulate methane monooxygenase (pMMO) in Methylococcus capsulatus (Bath) by acetylene.. BIOCHIMICA ET BIOPHYSICA ACTA-PROTEINS AND PROTEOMICS 2015, 1854(12), 1842-52.
- Chen Kelvin H.-C.*, Chuankhayan Phimonphan, Wu Hsin-Hui, Chen Chun-Jung, Fukuda Mitsuhiro, Yu Steve S.-F., Chan Sunney I. The bacteriohemerythrin from Methylococcus capsulatus (Bath): Crystal structures reveal that Leu114 regulates a water tunnel. Journal of Inorganic Biochemistry 2015, 150, 81-89.
- Nai-Ti Lin, Kamani Satyanarayana, Chih-Hsien Chen, Yi-Fang Tsai, Steve S.-F. Yu, Sunney I. Chan, Tien-Yau Luh Controlling the Orientation of Pendants in Two-Dimensional Comb-Like Polymers by Varying Stiffness of Polymeric Backbones. MACROMOLECULES 2014-09, 47(18), 6166-6172.
- Yao-Sheng Chen, Wen-I Luo, Chung-Ling Yang, Yi-Jung Tu, Chun-Wei Chang, Chih-Hsiang Chiang, Chi-Yao Chang, Sunney I. Chan, Steve S.-F. Yu* Controlled Oxidation of Aliphatic C-H bonds in Metallo-Monooxygenases: Mechanistic Insights Derived from Studies on Deuterated and Fluorinated Hydrocarbons (Early Career Focused Review, Cover Art). JOURNAL OF INORGANIC BIOCHEMISTRY 2014-05, 134, 118-133.
- Penumaka Nagababu, Steve S.-F. Yu, Suman Maji, Ravirala Ramu and Sunney I. Chan* Developing an efficient catalyst for controlled oxidation of small alkanes under ambient conditions (cover art). CATALYSIS SCIENCE AND TECHNOLOGY 2014-04, 4, 930-935.
- Wang DR, Hsiao JC, Wong CH, Li GC, Lin SC, Yu SSF, Chen W, Chang W, Tzou DL Vaccinia Viral Protein A27 is Anchored to the Viral Membrane via a Cooperative Interaction with Viral Membrane Protein A17.. JOURNAL OF BIOLOGICAL CHEMISTRY 2014-03, 289, 6639-6655.
- Peter P.-Y. Chen, Penumaka Nagababu, Steve S.-F. Yu, Sunney I. Chan* Development of the Tricopper Cluster as a Catalyst for the Efficient Conversion of Methane into MeOH. CHEMCATCHEM 2014-02, 6, 429-437.
- Yao-Sheng Chen, Wen-I Luo, Tsu-Lin Lee, Steve S.-F. Yu*, Chi-Yao Chang* Identification of the proteins required for fatty acid desaturation in zebrafish (Danio rerio). BIOCHEMICAL AND BIOPHYSICAL RESEARCH COMMUNICATIONS 2013-11, 440(4), 671-6.
- Chih-Hsiang Chiang, Ravirala Ramu, Yi-Jung Tu, Chung-Ling Yang, Kok Yaoh Ng, Wen-I Luo, Charles H. Chen, Yu-Ying Lu, Chen-Lun Liu, Steve S.-F. Yu* Regioselective Hydroxylation of C12-C15 Fatty Acids with Fluorinated Substituents by Cytochrome P450 BM3 (highlighted as a frontispiece of the issue). CHEMISTRY-A EUROPEAN JOURNAL 2013-10, 19 (41),13680-13691.
- Sunney I. Chan*, Yu-Jhang Lu, Penumaka Nagababu, Suman Maji, Mu-Cheng Hung, Marianne M. Lee, I-Jui Hsu, Pham Dinh Minh, Jeff C.-H. Lai, Kok Yoah Ng, Sridevi Ramalingam, Steve S.-F. Yu*, Michael K. Chan* Efficient Oxidation of Methane to Methanol by Dioxygen Mediated by Tricopper Clusters. ANGEWANDTE CHEMIE-INTERNATIONAL EDITION 2013-03, 52, 3731-3735.
- Minh D. Pham, Steve S.-F. Yu, Chau-Chung Han*, Sunney I. Chan* Improved mass spectrometric analysis of membrane proteins based on rapid and versatile sample preparation on nanodiamond particles.. ANALYTICAL CHEMISTRY 2013, 85(14), 6748-55.
- Penumaka Nagababu, Suman Maji, Manyam Praveen Kumar, Peter, P.-Y. Chen, Steve S.-F Yu*, Sunney I. Chan* Efficient room-temperature oxidation of hydrocarbons mediated by tricopper cluster complexes with different ligands. ADVANCED SYNTHESIS & CATALYSIS 2012, 354, 3275-3282.
- Suman Maji, Jason C.-M. Lee, Yu-Jhang Lu, Chang-Li Chen, Mu-Cheng Hung, Peter P.-Y. Chen, Steve S.-F. Yu, Sunney I. Chan* Dioxygen Activation of a Trinuclear CuICuICuI Cluster Capable of Mediating Facile Oxidation of Organic Substrates: Competition between O-Atom Transfer and Abortive Inter-cluster Reduction. CHEMISTRY-A EUROPEAN JOURNAL 2012, 18(13), 3955-3968.
- Feng-Chun Lo, Jyh-Fu Lee, Wen-Feng Liaw, I-Jui Hsu*, Yi-Fang Tsai, Sunney I. Chan, Steve S.-F. Yu* The Metal Core Structures in the Recombinant Escherichia coli Transcriptional Factor SoxR. CHEMISTRY-A EUROPEAN JOURNAL 2012, 18(9), 2565-2577.
- Ravirala Ramu, Chun-Wei Chang, Ho-Husan Chou, Li-Lan Wu, Chih-Hsiang Chiang, Steve S.-F. Yu* Regio-selective hydroxylation of gem-difluorinated octanes by alkane hydroxylase (AlkB). TETRAHEDRON LETTERS 2011-06, 52(23), 2950-2953.
- Wu Li-Lan, Yang Chung-Ling, Lo Feng-Chun, Chiang Chih-Hsiang, Chang Chun-Wei, Ng Kok Yaoh, Chou Ho-Hsuan, Hung Huei-Ying, Chan Sunney I., Yu Steve S.-F.* Tuning the Regio- and Stereoselectivity of C-H Activation in n-Octanes by Cytochrome P450 BM-3 with Fluorine Substituents: Evidence for Interactions Between a C-F Bond and Aromatic π Systems. Chemistry - A European Journal 2011-03, 17(17), 4774-4787.
- Sunney I. Chan*, H.-Hoa T. Nguyen, Kelvin H.-C. Chen, Steve S.-F. Yu* OVER-EXPRESSION AND PURIFICATION OF THE PARTICULATE METHANE MONOOXYGENASE FROM METHYLOCOCCUS CAPSULATUS (BATH). Methods in Enzymology 2011-03, 495, 177-193.
- Shih PC, Yang MS, Lin SC, Ho Y, Hsiao JC, Wang DR, Yu SS, Chang W, Tzou DL A turn-like structure "KKPE" segment mediates the specific binding of viral protein A27 to heparin and heparan sulfate on cell surfaces.. JOURNAL OF BIOLOGICAL CHEMISTRY 2009, 284(52), 36535-46.
- Mansoureh Mousavi, SteveS.-F.Yu, Der-LiiM.Tzou A 13C solid-state NMR analysis of vitamin D compounds. SOLID STATE NUCLEAR MAGNETIC RESONANCE 2009, 36, 24-31.
- Ng KY, Tu LC, Wang YS, Chan SI, Yu SS-F* Probing the hydrophobic pocket of the active site in the particulate methane monooxygenase (pMMO) from Methylococcus capsulatus (Bath) by variable stereoselective alkane hydroxylation and olefin epoxidation.. CHEMBIOCHEM 2008, 9(7), 1116-23.
- Lo F.-C., Chen C.-L., Lee C.-M., Tsai M.-C., Lu T.-T., Liaw W.-F., Yu S. S.-F.* A study of NO trafficking from dinitrosyl-iron complexes to the recombinant E. coli transcriptional factor SoxR.. JOURNAL OF BIOLOGICAL INORGANIC CHEMISTRY 2008, 13(6), 961-72.
- Kao WC, Wang VC, Huang YC, Yu SS, Chang TC, Chan SI Isolation, purification and characterization of hemerythrin from Methylococcus capsulatus (Bath).. JOURNAL OF INORGANIC BIOCHEMISTRY 2008, 102(8), 1607-14.
- Chan SI, Yu SS Controlled oxidation of hydrocarbons by the membrane-bound methane monooxygenase: the case for a tricopper cluster.. ACCOUNTS OF CHEMICAL RESEARCH 2008, 41(8), 969-79.
- Yu SS, Ji CZ, Wu YP, Lee TL, Lai CH, Lin SC, Yang ZL, Wang VC, Chen KH, Chan SI The C-terminal aqueous-exposed domain of the 45 kDa subunit of the particulate methane monooxygenase in Methylococcus capsulatus (Bath) is a Cu(I) sponge.. BIOCHEMISTRY 2007, 46(48), 13762-74.
- Chan SI, Wang VC, Lai JC, Yu SS, Chen PP, Chen KH, Chen CL, Chan MK Redox potentiometry studies of particulate methane monooxygenase: support for a trinuclear copper cluster active site.. ANGEWANDTE CHEMIE-INTERNATIONAL EDITION 2007, 46(12), 1992-4.
- Tseng, CF, Burger, A, Mislin, GLA, Schalk, IJ, Yu, SSF, Chan, SI and Abdallah, MA Bacterial siderophores: the solution stoichiometry and coordination of the Fe(III) complexes of pyochelin and related compounds. JOURNAL OF BIOLOGICAL INORGANIC CHEMISTRY 2006-06, 11(4), 419-432.
- Kao WC, Chen YR, Yi EC, Lee H, Tian Q, Wu KM, Tsai SF, Yu SS, Chen YJ, Aebersold R, Chan SI Quantitative proteomic analysis of metabolic regulation by copper ions in Methylococcus capsulatus (Bath).. J. Biol. Chem. 2004, 279(49), 51554-60.
- S.-C. Hung, C.-Li. Chen, K.H.-C. Chen, S.S.-F. Yu and S.I. Chan* The catalytic copper clusters of the particulate methane monooxygenase from methanotrophic bacteria: Electron paramagnetic resonance spectral simulations.. JOURNAL OF THE CHINESE CHEMICAL SOCIETY 2004, 51, 1229-1244.
- S.I. Chan*, K.H.C. Chen, S.S.F. Yu, C.L. Chen and S.S.J. Kuo Toward delineating the structure and function of the particulate methane monooxygenase from methanotrophic bacteria.. Biochemistry 2004, 43, 4421-4430.
- M.S. Vinchurkar, K.H.C. Chen, S.S.F. Yu, S.J. Kuo, H.C. Chiu, S.H. Chien and S.I. Chan* Polarized ATR-FTIR spectroscopy of the membrane-embedded domains of the particulate methane monooxygenase.. Biochemistry 2004, 43, 13283-13292.
- C.L. Chen, K.H.C. Chen, S.C. Ke, S.S.F. Yu and S.I. Chan* Preparation and characterization of a (Cu, Zn)-pMMO from Methylococcus capsulatus (Bath).. J. Inorg. Biochem. 2004, 98, 2125-2130.
- K. H.-C. Chen, C.-L. Chen, C.-F. Tseng, S.S.-F. Yu, S.-C. Ke, J.-F. Lee, H.T. Nguyen, S.J. Elliott, J.O. Alben and S.I. Chan* The copper clusters in the particulate methane monooxygenase (pMMO) from Methylococcus capsulatus (Bath) .. JOURNAL OF THE CHINESE CHEMICAL SOCIETY 2004, 51, 1081-1098.
- S.S.F. Yu, L.Y. Wu, K.H.C. Chen, W.I. Luo, D.S. Huang, S.I. Chan* The stereospecific hydroxylation of [2,2-2h2]butane and chiral dideuteriobutanes by the particulate methane monooxygenase from Methylococcus capsulatus (Bath).. J. Biol. Chem. 2003, 278, 40658-40669.
- S.S.F. Yu, L.Y. Wu, K.H.C. Chen, M.Y.H. Tseng, Y.S. Wang, C.F. Tseng, Y.J. Chen, D.S. Huang and S.I. Chan* Production of high quality pMMO in high yields from Methylococcus capsulatus (Bath) with a hollow-fiber membrane bioreactor.. J. Bacteriol. 2003, 185, 5915-5924.
- Chih-Cheng Liu, Yi-Fang Tsai, Wondemagegn H. Wanna, Ravirala Ramu, Damodar Janmanchi, Sunney I. Chan, Steve S.-F. Yu*, 2019, “Selective Oxidation of Alkanes by Metallo-monooxygenase and Their Nano-biomimetics”, editor(s): Armando J.L. Pombeiro , M. Fátima C. Guedes da Silva, Alkane Functionalization, pp. chapter 15, Chichester: John Wiley & Sons Ltd.
- 俞聖法、羅鳳君、洪木成、廖文峰、蔡銘哲、魯才德,2009,〈生物系統中的鐵硫簇活性中心蛋白與亞硝基鐵硫錯合物〉,《化學》,第六十七卷第二期,頁133-141。。
- 李耀昌、陳俊榮、馮學深、李志甫、黃玉山、李明道、宋豔芳、鄭有舜、江素玉、陳慶曰、黃佩瑜、許益瑞、俞聖法、吳文桂,2008,〈同步輻射在生命科學上的應用與發展〉,《物理雙月刊》,第30卷第1期,頁8-26。
- Chan, K. H.-C.; Yu, S. S.-F.; Chan, S. I., 2005, “XAS Studies of pMMO from Methylococcus capsulatus (Bath)”, NSRRC Activity Report, pp. 65.
- 2. 陳耀甥、張鈞崴、楊宗霖、吳麗嵐、江志祥、拉姆、羅文一、陳長謙、俞聖法,2013,〈金屬單加氧酵素內其活化烷烴基質選擇性的控制〉,《化學季刊》,第71卷第1期,頁67-81。
- 透過氧化苯生產對位苯醌的方法、由苯製造1,4-對苯二酚的方法及銅奈米粒子作為催化劑的用途
Taiwan: I 679190 2019 - 可催化碳氫化合物氧化之分子催化劑與氧化碳氫化合物的方法 Molecular Catalyst Capable of Catalyzing Oxidation of Hydrocarbons and Methods for Oxidizing Hydrocarbons
USA-regular: 10,005,746 2018 - 可催化碳氫化合物氧化之分子催化劑與氧化碳氫化合物的方法 Molecular Catalyst Capable of Catalyzing Oxidation of Hydrocarbons and Methods for Oxidizing Hydrocarbons
Taiwan: I625164 2018


